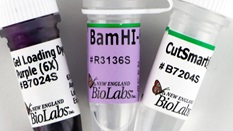
BamHI
BamHI has been reformulated with Recombinant Albumin (rAlbumin) beginning with Lot #10184085. Learn more.
We are excited to announce that all reaction buffers are now BSA-free. NEB began switching our BSA-containing reaction buffers in April 2021 to buffers containing Recombinant Albumin (rAlbumin) for restriction enzymes and some DNA modifying enzymes. Find more details at www.neb.com/BSA-free.
- Time-Saver™ qualified for digestion in 5-15 minutes
- High Fidelity (HF®) version available (NEB #R3136) supplied with rCutSmart™ Buffer
- Supplied with 1 vial of Gel Loading Dye, Purple (6X)
- Restriction Enzyme Cut Site: G/GATCC
Featured Video
-
Product Information
BamHI has a High Fidelity version BamHI-HF® (NEB #R3136).
High Fidelity (HF) Restriction Enzymes have 100% activity in rCutSmart Buffer; single-buffer simplicity means more straightforward and streamlined sample processing. HF enzymes also exhibit dramatically reduced star activity. HF enzymes are all Time-Saver qualified and can therefore cut substrate DNA in 5-15 minutes with the flexibility to digest overnight without degradation to DNA. Engineered with performance in mind, HF restriction enzymes are fully active under a broader range of conditions, minimizing off-target products, while offering flexibility in experimental design.
For details on NEB’s quality controls for restriction endonucleases, visit our Restriction Enzyme Quality page.Product Source
A E. coli strain that carries the BamHI gene from Bacillus amyloliquefaciens H (ATCC 49763).- This product is related to the following categories:
- Restriction Endonucleases B Products,
- Time-Saver Qualified Restriction Enzymes Products
- This product can be used in the following applications:
- Fast Cloning: Accelerate your cloning workflows with reagents from NEB,
- Restriction Enzyme Digestion
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
-

Reduce Star Activity with High-Fidelity Restriction Enzymes
-

TIME-SAVER™ Protocol for Restriction Enzyme Digests
-

NEB® TV Ep. 15 – Applications of Restriction Enzymes
-

Restriction Enzyme Digest Protocol: Cutting Close to DNA End
-

Restriction Enzyme Digestion Problem: DNA Smear on Agarose Gel
-
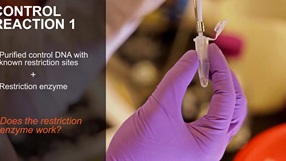
Why is My Restriction Enzyme Not Cutting DNA?
-

Restriction Enzyme Digest Problem: Too Many DNA Bands
-
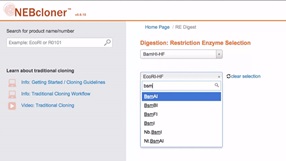
Double Digestion with NEBcloner
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.