Restriction Enzyme Digestion
Subcloning requires the use of 1-2 restriction enzymes that cut immediately outside the insert fragment without cutting within the insert itself. Restriction enzymes that have a recognition site within the multiple cloning site (MCS) are commonly used since they do not cut elsewhere in the vector DNA and typically produce two easily resolved DNA fragments. The gene of interest is most commonly subcloned into an expression vector for improved protein expression and/or addition of a purification tag. In this case, it is essential that the gene be inserted in the correct orientation and in frame with the transcription promoter.
The Polymerase Chain Reaction (PCR) is commonly used to amplify a gene or DNA fragment of interest, from any source of DNA, to be cloned. In order to generate compatible ends, it is common to add restriction sites to the 5’ end of both PCR primers. When adding restriction sites to a PCR primer, it is recommended to include 6 bases between the recognition site and the 5’ end of the primer. These additional bases provide sufficient DNA for the restriction enzyme to bind the recognition site and cut efficiently. When selecting a restriction site(s) to add to the primers, it is important to determine which site(s) will be compatible with your selected vector, whether directional cloning is desired and, most importantly, confirm that the recognition site(s) does not occur within the gene or DNA fragment.
Choose Type:
- Removal of Single-Stranded Extension Protocol using Mung Bean Nuclease (M0250)
- Standard Digest Using RE-Mix®
- Double Digest Protocol using Two RE-Mix® Enzymes
- Optimizing Restriction Endonuclease Reactions
- Protocol for Cre Recombinase (M0298)
- Double Digest Protocol using One RE-Mix and One Standard Restriction Enzyme
- Protocol for Glucosylation and digestion of Genomic DNA using AbaSI (#R0665)
- Protocol for Direct Digestion of gDNA during droplet digital PCR (ddPCR)
- Double Digest Protocol with Standard Restriction Enzymes
-
Restriction Enzymes at NEB: Over 30 years of Innovation
-
Restriction Endonucleases: Molecular Cloning and Beyond
-
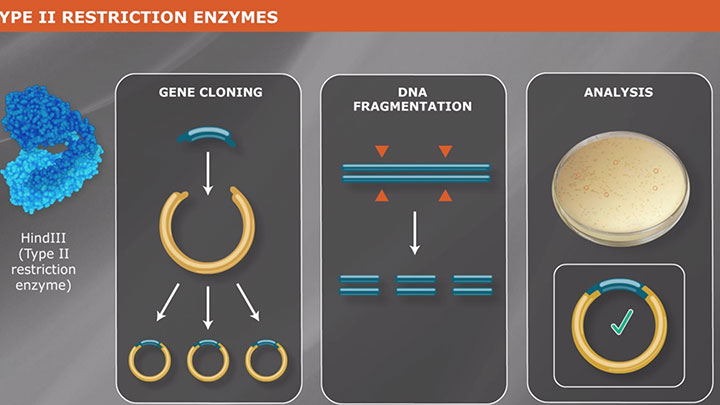
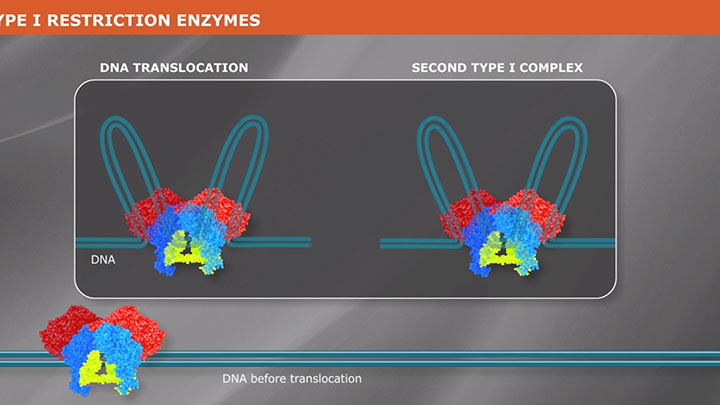
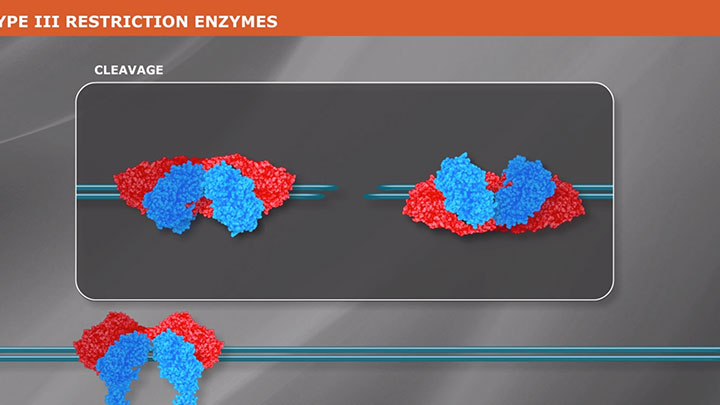
Type II Restriction Enzymes: What You Need to Know | NEB
Read about Type II restriction enzymes and the distinguishing properties of the four principle subtypes.
-
A Modern Day Gene Genie Sir Richard Roberts on Rebase
-
Whole genome assembly from next generation sequencing data using restriction and nicking enzymes in optical mapping and proximity-based ligation strategies
High throughput sequencing methods have revolutionized genomic analysis by producing millions of sequence reads from an organism’s DNA at an ever decreasing cost.
- Molecular Cloning Technical Guide
- Alphabetized List of Recognition Sequences
- Cleavage Of Supercoiled DNA
- Compatible Cohesive Ends and Generation of New Restriction Sites
- Dam-Dcm and CpG Methylation
- Enzymes with Multiple Recognition Sequences
- Enzymes with Nonpalindromic Sequences
- Frequencies of Restriction Sites
- Interrupted Palindromes
- Isoelectric Points (pI) for Restriction Enzymes
- Isoschizomers
- Recleavable Blunt Ends
- Recleavable Filled-in 5' Overhangs
- Why Choose Recombinant Enzymes?
- Restriction Enzyme Troubleshooting Guide
- Troubleshooting Guide for Cloning
- Activity at 37°C for Restriction Enzymes with Alternate Incubation Temperatures
- Alteration of Apparent Recognition Specificities Using Methylases
- Cleavage Close to the End of DNA Fragments
- Digestion of Agarose-Embedded DNA: Info for Specific Enzymes
- Double Digests
- Heat Inactivation
- NEBuffer Activity/Performance Chart with Restriction Enzymes
- Optimizing Restriction Endonuclease Reactions
- Restriction Endonucleases - Survival in a Reaction
- Restriction Enzyme Tips
- Restriction Enzymes for Droplet Digital PCR (ddPCR)
- Site Preferences
- Star Activity
- Traditional Cloning Quick Guide
Feature Articles
Brochures
Selection Tools
Troubleshooting Guides
Usage Guidelines
- Fu YB, Peterson G. W., Dong Y (2016) Increasing Genome Sampling and Improving SNP Genotyping for Genotyping-by-Sequencing with New Combinations of Restriction Enzymes G3 (Bethesda); 6:4, 845-846. PubMedID: 26818077
- Shah, S., Sanchez, J., Stewart, A., et al. (2015) Probing the Run-On Oligomer of Activated SgrAI Bound to DNA PLoS One; 10(4), PubMedID: 25880668, DOI: 10.1371/journal.pone.0124783.
- Loenen, W.A., Raleigh, E.A. (2014) The other face of restriction: modification-dependent enzymes. Nucleic Acids Res; 42, 56-69. PubMedID: 23990325
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.