
Quality at NEB
- NEB’s quality certifications (ISO 13485:2016 and ISO 9001:2015), quality assurance practices, and GMP-grade* manufacturing
- Product lifecycle tracking practices (expiration dating and barcoding)
- Industry-leading quality controls
- Examples of product quality across multiple vendors
For questions about NEB’s quality processes for standard and custom products, please contact info@neb.com.
NEB holds both ISO 13485: 2016 and ISO 9001: 2015 certifications at its facilities in Ipswich, Rowley and Beverly, MA (USA). Learn more about our ISO certifications on our Certifications page.
To better serve the needs of customers in regulated markets, NEB has opened a state-of-the-art 43,000 sq. ft. production facility in Rowley, MA for the manufacture of GMP-grade* materials – approximately 15 minutes from our main campus in Ipswich, MA, USA. This purpose-built facility includes Quality Control and Production functions ranging from a shipping/receiving area and dedicated warehouse, to separate inoculation preparation, fermentation, purification and fill suites.
- Product Certificates of Origin are available upon request.
- Established validation programs for:
- Change Management and Customer Notification of Changes
- Use of a validated 21CFR Part 11-compliant electronic Quality Management Software (eQMS) for Quality records and document control
- NEB has been recognized for the quality of its products; most recently, NEB was awarded a Life Science Industry Award® (LSIA) for its “Molecular Biology Products”, citing both product purity and performance. Additional details can be found in our press release.
- Case studies (>40 available upon request) of the use of NEB products in regulated applications are available. NEB reagents are currently used in the following regulated applications:
-
– Specialized products (e.g., NEBNext® Library Preparation kits)
– Shipping process
– Automated filling lines
– Product storage chambers
– Critical process equipment
-
– Molecular Diagnostics
– Food Safety Testing
– Animal Health
– Pharmaceutical QC reagents and starting materials
New England Biolabs now has a policy of assigning expiration dates to many of our enzymes and reagents. This policy is designed to satisfy the needs of a growing number of our customers who require such information to meet their regulatory requirements. For such customers, the expiration date indicates the period of time over which NEB will guarantee 100% activity of the enzyme if stored under the recommended storage conditions.*
Our expiration dates have been developed as a result of many years of experience with our products and represent a minimum guaranteed life expectancy for full activity of a given product. It is not the intent of this dating policy to imply that a product will necessarily lose activity after the expiration date. In fact, many of our enzymes will retain significant, if not full, activity for many months or even years after the expiration date. For many basic research applications, enzyme vials may be suitable for use well past their stated expiration date. Disposal and or continued use of enzymes past their expiration dates should be at the discretion of the end user.
* Enzymes should be stored long-term at the recommended temperature (most often -20°C; some enzymes have alternate recommended storage temperatures, such as -80°C). During use, enzymes should be stored on ice, and then returned to their long term storage conditions promptly after use.
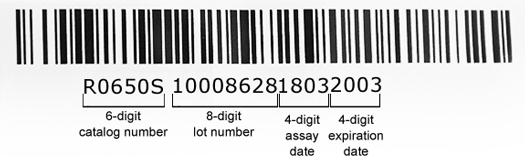
The barcode (see image below) is based on Code 128 symbology, consists of a six-digit catalog number (eg., R0650S), an eight-digit lot number (eg., 10008628), a four-digit assay date (eg., 1803), and a four-digit expiration date (eg., 2003). For the four pieces of coded information (cat #, lot #, assay date, exp. date), the details are as follows:
- catalog # - the standard six-digit system is our standard catalog number.
- lot # - NEB utilizes a system-assigned, non-intelligent lot number to identify every unique packaging run. This lot number is printed on the outside of the package and embedded in the package barcode.
- assay date - this is a four-digit sequence, YYMM; for example, 1803 would indicate an assay date of March 2018.
- expiration date - this is a four-digit sequence, YYMM; for example, 2003 would indicate an expiration date of March 2020.
A range of standard Quality Controls are run for each product.
- Stability testing methodology includes:
- New product shelf life is set based on accelerated testing, performance of similar products, and verified by real-time testing.
- Fully packaged retains are stored under controlled conditions for one year past expiration date.
- Proprietary methods are developed by Research, Applications and Product Development scientists, and verified by Product Managers and the Quality Control lab.
- Availability of Certificates of Analysis and Product Specifications for custom or catalog products
- Lot-to-lot reproducibility is maintained by concentration measurement, activity measurement and functional testing.
Extreme purity with NEB's T4 DNA Ligase
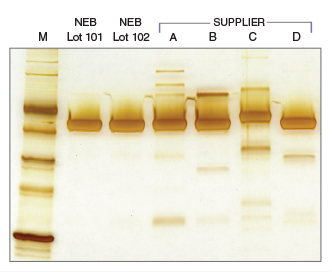
T4 DNA Ligase Competitor Study - Nuclease Contamination
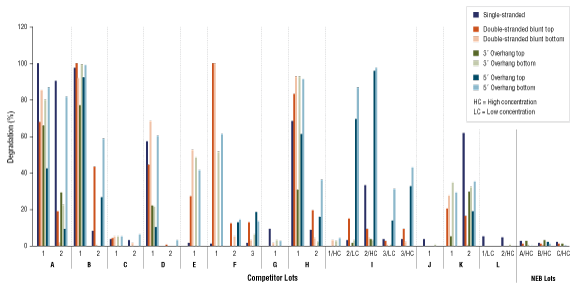
Restriction Enzyme Competitor Study: Nuclease Contamination
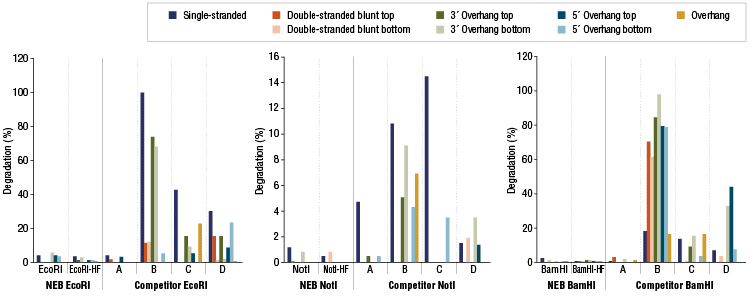
EcoRI, NotI, and BamHI from multiple suppliers were tested in reactions containing a fluorescent labeled single stranded, double stranded blunt, 3’overhang or 5’ overhang containing oligonucleotides. The percent degradation is determined by capillary electrophoresis and peak analysis. The resolution is at the single nucleotide level.


