-
Product Information
High Fidelity (HF) Restriction Enzymes have 100% activity in rCutSmart Buffer; single-buffer simplicity means more straightforward and streamlined sample processing. HF enzymes also exhibit dramatically reduced star activity. HF enzymes are all Time-Saver qualified and can therefore cut substrate DNA in 5-15 with the flexibility to digest overnight without degradation to DNA. Engineered with performance in mind, HF restriction enzymes are fully active under a broader range of conditions, minimizing off-target products, while offering flexibility in experimental design.
NEB extensively performs quality controls on all standard and high-fidelity (HF) restriction enzymes. Examples of nuclease contamination studies for some of our HF restriction enzymes are shown below.
Restriction Enzyme Competitor Study: Nuclease Contamination
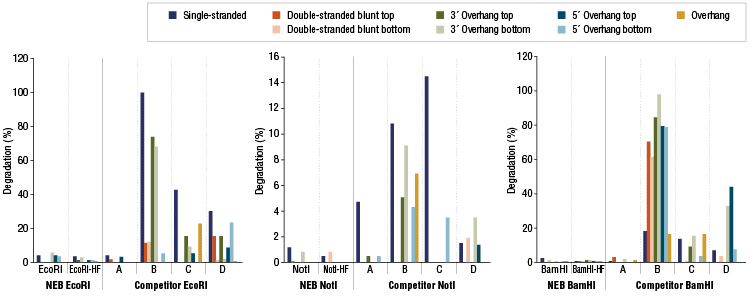
EcoRI, NotI, and BamHI from multiple suppliers were tested in reactions containing a fluorescent labeled single stranded, double stranded blunt, 3’overhang or 5’ overhang containing oligonucleotides. The percent degradation is determined by capillary electrophoresis and peak analysis. The resolution is at the single nucleotide level.Product Source
An E. coli strain that carries the cloned and modified SbfI gene from Streptomycesspecies Bf-61 (S.K. Degtyarev).- This product is related to the following categories:
- Restriction Endonucleases S Products,
- High-Fidelity (HF®) Restriction Endonucleases Products,
- Time-Saver Qualified Restriction Enzymes Products
- This product can be used in the following applications:
- Fast Cloning: Accelerate your cloning workflows with reagents from NEB,
- Restriction Enzyme Digestion
Featured Videos
-

Reduce Star Activity with High-Fidelity Restriction Enzymes
-

TIME-SAVER™ Protocol for Restriction Enzyme Digests
-

NEB® TV Ep. 15 – Applications of Restriction Enzymes
-

Restriction Enzyme Digest Protocol: Cutting Close to DNA End
-

Restriction Enzyme Digestion Problem: DNA Smear on Agarose Gel
-
Why is My Restriction Enzyme Not Cutting DNA?
-

Restriction Enzyme Digest Problem: Too Many DNA Bands
-
Double Digestion with NEBcloner
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.
Choose your country
North America
Europe
Asia-Pacific

Find a Golden Butterfly for a Chance to Win!
Help us celebrate our 50th anniversary! We have hidden 1,000 golden butterflies and are waiting for you to find them. They can be anywhere that you find NEB! Beginning April 15th, be sure to visit our website and tables at tradeshows and events you are attending. Visit our social media channels frequently for tips on where we have hidden the butterflies – and once you find one, either click or scan the code to be eligible for a 50th anniversary prize pack, as well as a grand prize trip to NEB headquarters in Ipswich, MA!.

To save your cart and view previous orders, sign in to your NEB account. Adding products to your cart without being signed in will result in a loss of your cart when you do sign in or leave the site.