DNA Ligase Products
Learn more about NEB's quality controls for DNA ligases.
For help with selecting or using a DNA ligase, visit our supporting materials:Ligase Fidelity
Extensive research has been conducted at NEB regarding the end-joining ligase fidelity (discrimination against ligating mismatched overhangs) and bias (sequence preference) for short, cohesive ends. This information can be used to optimize design of Golden Gate Assembly and achieve high efficiency and high fidelity assemblies of 35+ fragments.
NEB has compiled this information into the following tools:
Ligase Fidelity Viewer™ (v2)
Visualize overhang ligation preferences
GetSet™
Predict high-fidelity junction sets
SplitSet™
Split DNA sequence for scarless high-fidelity assembly
For more information on ligase fidelity and Golden Gate assembly, watch this webinar or visit neb.com/goldengate.
SilverXpress™ is a trademark of Life Technologies, Inc.
Choose Type:
- Ligation Protocol with T4 DNA Ligase (M0202)
- NEBNext Quick Ligation Module Protocol (E6056)
- Quick Ligation Protocol (M2200)
- Transformation Protocol
- Transformation Protocol (M0367)
- Transformation Protocol (M0370)
- Ligation Protocol for Cloning with Instant Sticky-end Ligase Master Mix (M0370)
- Ligation Protocol for Cloning with Blunt/TA Ligase Master Mix (M0367)
- Ligation Protocol for Cloning with ElectroLigase® (M0369)
- Transformation Protocol (M0369)
- Protocol for ssDNA/RNA Ligation (NEB #M0319)
- Ligation Protocol with T3 DNA Ligase (M0317)
- Ligation Protocol with T7 DNA Ligase (M0318)
- Ligation protocol using SplintR® Ligase (NEB #M0375)
- E. coli DNA Ligase Protocol (M0205)
- Protocol for 9°N DNA Ligase (M0238)
- Protocol for Taq DNA Ligase (M0208)
- HiFi Taq DNA Ligase (M0647) Protocol
- Protocol for Salt-T4® DNA Ligase (NEB #M0467)
- Protocol for Hi-T4™ DNA Ligase (NEB #M2622)
- DNA Ligase Selection Chart
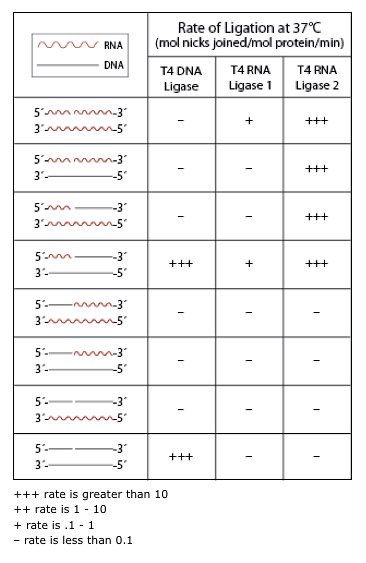
- Properties of DNA and RNA Ligases
- Troubleshooting Guide for Cloning
- Troubleshooting Guide for Ligases
- Troubleshooting Tips for Ligation Reactions
- Tips for Maximizing Ligation Efficiencies
- Traditional Cloning Quick Guide
Selection Tools
Troubleshooting Guides
Usage Guidelines
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.