Endoproteinase AspN
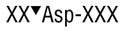
Endoproteinase AspN (flavastacin) is a zinc metalloendopeptidase which selectively cleaves protein and peptide bonds N-terminal to aspartic acid residues.
- Free of glycerol and detergents which may interfere with mass spectrometry or liquid chromatography methods.
- Supplied in dry format
- Optimal activity and stability for up to 12 months

-
Product Information
Endoproteinase AspN (flavastacin) is a zinc metalloendopeptidase which selectively cleaves peptide bonds N-terminal to aspartic acid residues(1).
Product Source
Purified from Flavobacterium menigosepticum.Reconstitution
Endoproteinase AspN should be reconstituted by the addition of 50-500 µl of high purity water. Rapid autolysis is a function of enzyme concentration.- This product is related to the following categories:
- Proteases Products,
- Proteome Analysis Products
- This product can be used in the following applications:
- Glycomics and glycoproteomics,
- Proteomics
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.