Phusion® High-Fidelity DNA Polymerase
For high speed and high performance PCR. Manufactured and quality-controlled at New England Biolabs, Thermo Scientific® Phusion High-Fidelity DNA Polymerase offers both high fidelity and robust performance.
- 50X higher fidelity than Taq
- Robust reactions - maximal success with minimal optimization
- Offered with multiple buffers for customized reaction setup for different DNA templates
-
Product Information
High-Fidelity DNA Polymerases are important for applications in which the DNA sequence needs to be correct after amplification. Its unique structure, a novel Pyrococcus-like enzyme fused with a processivity-enhancing domain, increases fidelity and speed. Phusion DNA Polymerase is an ideal choice for cloning and can be used for long or difficult amplicons. With an error rate > 50-fold lower than that of Taq DNA Polymerase and 6-fold lower than that of Pyrococcus furiosus DNA Polymerase (1), Phusion is one of the most accurate thermostable polymerases available. Phusion DNA Polymerase possesses 5´→ 3´ polymerase activity, 3´→ 5´ exonuclease activity and will generate blunt-ended products.
Phusion DNA Polymerase is supplied with standard 5X Phusion HF Buffer, as well as 5X Phusion GC Buffer, which can be used for complex or GC-rich templates. Each of these buffers contains MgCl2 (1.5 mM at the final [1X] reaction concentration). Reactions can also be optimized using the provided DMSO or MgCl2 solutions.
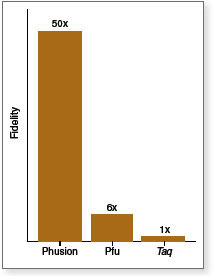
Fidelity assays were performed using a lacI-based method modified from Frey & Suppman, 1995. 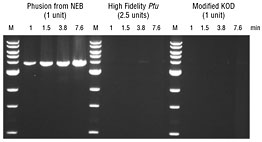
A 3.8 kb fragment was amplified from 50 ng of Jurkat gDNA using different polymerases. Reactions were carried out according to the manufacturer's recommended conditions. Extension times are indicated (in minutes). Ladder L is a 1 kb DNA Ladder (NEB# N3232). Product Source
An E. coli strain that carries the Phusion DNA Polymerase gene.- This product is related to the following categories:
- Phusion® High-Fidelity DNA Polymerases Products
- This product can be used in the following applications:
- Gibson Assembly®,
- Long Range PCR,
- Fast PCR,
- High-Fidelity PCR,
- Multiplex PCR,
- Specialty PCR,
- Routine PCR, PCR
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.