PNGase F – Designed for your application
The industry standard for purity
< Return to "PNGase F Products"
Highlights
NEB is certified to both ISO 9001:2008 and ISO 13485:2003 standards for quality management systems. |
 |
- Recombinant enzymes; manufactured in-house
- All glycosidase products from NEB exhibit ≥95% purity, as determined by SDS-PAGE and intact ESI-MS
- Stringent quality control testing includes assays for detecting contaminating exoglycosidase, endoglycosidase and proteolytic activity
Quality data
Every NEB enzyme is subjected to a quality control assay to determine protein purity. The physical purity is assessed by both SDS-PAGE and intact ESI-MS analysis. An example of this is shown below, using PNGase F. It is imperative to hold these enzymes to a high quality standard, as they must be compatible with the needs of regulated applications and standardized procedures, as well being free of contaminants and side activities. High-quality products are also necessary to meet challenges associated with glycoproteomic analyses.- Download our latest white paper “Redesigning glycosidase manufacturing quality for pharmaceutical and clinical applications”
- Learn more about NEB's quality philosophy.
- View our press release about discussing our plans for a GMP facility.
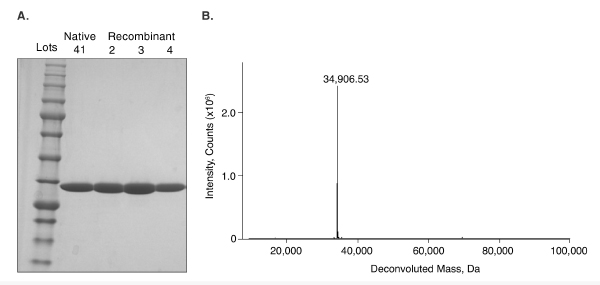
Lane 1: 5 μl Color Prestained Protein Standard (11-245 kDa) (NEB #P7712)
Lane 2: 5 μl PNGase F (Glycerol Free), Lot 41 (NEB #P0705)
Lane 3: 5 μl PNGase F (Glycerol Free), Recombinant, Lot 2 (NEB #P0709)
Lane 4: 5 μl PNGase F (Glycerol Free), Recombinant, Lot 3 Lane 5: 5 μl PNGase F (Glycerol Free), Recombinant, Lot 4
(B) PNGase F (Glycerol Free), Recombinant (NEB# P0709), Lot 2. MW: 34,906.53 Daltons. Mass determination by an Agilent 6210 TOF LC/MS.
One or more of these products are covered by patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc. For more information, please email us at gbd@neb.com. The use of these products may require you to obtain additional third party intellectual property rights for certain application.

