ElectroLigase®
ElectroLigase® combines T4 DNA Ligase and an optimized, ready-to-use 2X reaction buffer containing a proprietary ligation enhancer and no PEG. This combination is specifically formulated to promote robust ligation of all types of DNA ends (e.g., blunt, sticky, TA). It is directly compatible with electrocompetent cells used for transformation by electroporation, without desalting or purification.
- Directly compatible with electrocompetent cells
- No thawing needed as it maintains liquid form at -20°C
- Not sure which ligase to choose? Refer to our DNA and RNA Ligase Properties Chart or DNA Ligase Selection Chart
Featured Video
-
Product Information
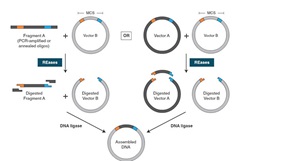
ElectroLigase™ combines T4 DNA ligase and an optimized, ready-to-use 2X reaction buffer containing a proprietary ligation enhancer and no PEG. This combination is specifically formulated to promote robust ligation of all types of DNA ends (blunt, sticky, TA). It is directly compatible, without desalting or purification, with electrocompetent cells used for transformation by electroporation. No thawing of the buffer is required as it maintains a liquid state during storage at -20°C*, thereby simplifying reaction set-up. By providing an optimized ratio of enzyme and buffer components, users are able to rapidly ligate all types of DNA ends applying a short incubation time at room temperature. Ligations for subcloning can be carried out in small volumes with low concentrations, allowing users to conserve precious DNA samples. These reactions can be pipetted directly, without purification or dilution, to transform many strains of electrocompetent E. coli**.
* Freezers vary in their actual internal temperatures. Our testing demonstrates that the enzyme and buffer remain liquid at -20°C.
**ElectroLigase is also compatible with chemically competent strains of E. coli. Performance is generally around 50% efficiency, when compared to the Blunt/TA Ligase Master Mix (NEB #M0367 ).
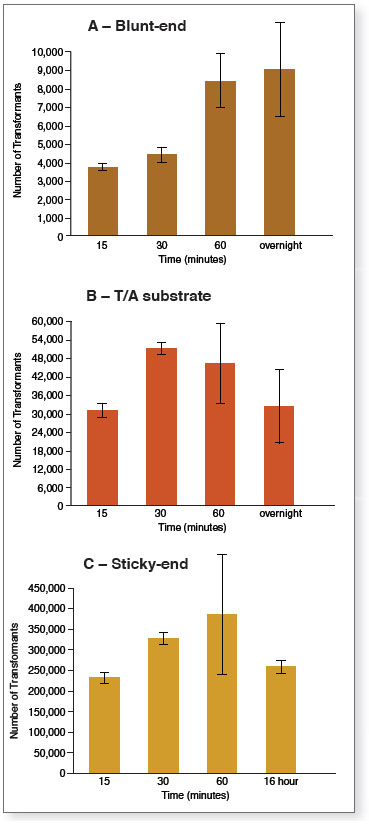
Ligation reactions containing equal amounts (20 ng vector and 3-fold molar excess of insert) of blunt- (A), T/A (B), or sticky-end (C) vector/insert pairs were set up using ElectroLigase and incubated for the times shown. After heat inactivation, 2 μl of each reaction were withdrawn and directly used to transform NEB 10-beta Electrocompetent E. coli (NEB #C3020). 50 μl aliquots of the outgrowth (diluted, in some cases) were plated onto selective plates and incubated overnight at 37°C. Colonies were counted, adjusted for plating dilution, and graphed.
Product Source
Purified from an E. coli strain containing a recombinant gene encoding T4 DNA Ligase.- This product is related to the following categories:
- DNA Ligases Products
- This product can be used in the following applications:
- Cloning Ligation
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.