Antarctic Phosphatase
Antarctic Phosphatase (AnP) is a heat labile alkaline phosphatase purified from a recombinant source.
- Rapid and irreversible heat inactivation eliminates unwanted activity
- Flexible reaction conditions (active in any restriction enzyme buffer, no clean-up required)
- No need for multiple phosphatases (AnP removes 5´- and 3´- phosphates from DNA, RNA and dNTPs)
- Active on unincorporated dNTPs in PCR products - improves DNA sequencing and SNP analysis
Featured Video
-
Product Information
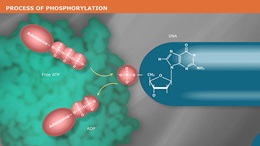
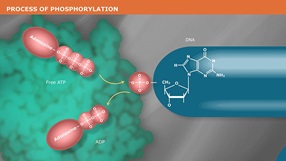
Antarctic Phosphatase (AnP) is a heat labile alkaline phosphatase purified from a recombinant source. AnP nonspecifically catalyzes the dephosphorylation of 5´ and 3´ ends of DNA and RNA phosphomonoesters. Also, AnP Hydrolyses ribo-, as well as deoxyribonucleoside triphosphates (NTPs and dNTPs). AnP is useful in many molecular biology applications such as the removal of phosphorylated ends of DNA and RNA for subsequent use in cloning or end-labeling of probes. In cloning, dephosphorylation prevents religation of linearized plasmid DNA. The enzyme acts on 5´ protruding, 5´ recessed, and blunt ends. AnP may also be used to degrade unincorporated dNTPs in PCR reactions to prepare templates for DNA sequencing or SNP analysis. AnP is completely and irreversibly inactivated by heating at 80°C for 2 minutes, thereby making removal of AnP prior to ligation or end-labeling unnecessary.
Product Source
An E. coli strain that carries the TAB5 AP gene, originally cloned in plasmid pNI (2), recloned in plasmid pEGTAB7-4.1(3).- This product is related to the following categories:
- Phosphatases Products,
- RNA Modification
- This product can be used in the following applications:
- Dephosphorylation
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.