Fpg
- Formamidopyrimidine DNA Glycosylase
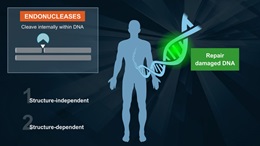
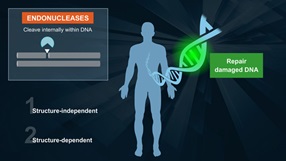
- Bifunctional DNA glycosylase with DNA N-glycosylase and AP lyase activities
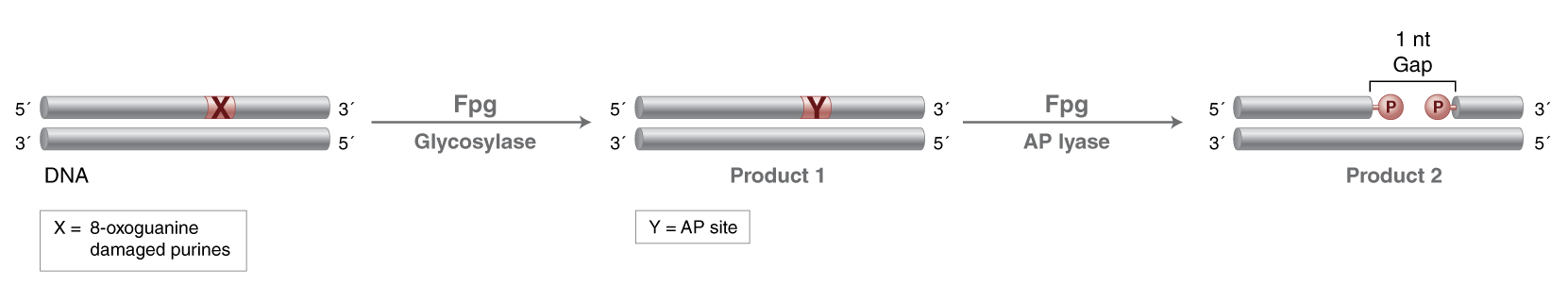
- N-glycosylase activity releases damaged purines, including 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) and 8-oxo-7,8-dihydroguanine (8-oxoG), generating an AP site. The AP lyase activity cleaves an AP site, via β and δ-elimination, creating a 1 nucleotide DNA gap with 5' and 3' phosphate termini.
Featured Video
-
Product Information
Fpg (formamidopyrimidine [fapy]-DNA glycosylase) (also known as 8-oxoguanine DNA glycosylase) acts both as a N-glycosylase and an AP-lyase. The N-glycosylase activity releases damaged purines from double stranded DNA, generating an apurinic (AP site). The AP-lyase activity cleaves both 3´ and 5´ to the AP site thereby removing the AP site and leaving a 1 base gap. Some of the damaged bases recognized and removed by Fpg include 7, 8-dihydro-8-oxoguanine (8-oxoguanine), 8-oxoadenine, fapy-guanine, methy-fapy-guanine, fapy-adenine, aflatoxin B1-fapy-guanine, 5-hydroxy-cytosine and 5-hydroxy-uracil (1,2).
Product Source
An E.coli strain that carries the cloned fpg gene (3)- This product is related to the following categories:
- DNA Repair Enzymes and Structure-specific Endonucleases Products
- This product can be used in the following applications:
- Polymerases for DNA Manipulation
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.