Trypsin-ultra™, Mass Spectrometry Grade
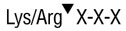
Trypsin-ultra, Mass Spectrometry Grade is a serine endopeptidase, which selectively cleaves peptide bonds C-terminal to lysine and arginine residues. Trypsin-ultra cleaves at Lys-Pro and Arg-Pro bonds at a much slower rate than when Lys and Arg are N-terminal to other residues.
- Analyze complex proteomes with minimal autolysis
- Compatible for simultaneous co-digestion with PNGase F (NEB# P0709)
- Compatible for simultaneous co-digestion with Endoproteinase LysC (NEB# P8109)
- Suitable for both in-gel and solution digests
- Supplied in dry format
- Optimal activity and stability for up to 12 months

-
Product Information
Trypsin-ultra™, Mass Spectrometry Grade is a serine endopeptidase. It selectively cleaves peptide bonds C-terminal to lysine and arginine residues (1). Trypsin-ultra is treated with L-(tosylamido-2-phenyl) ethyl chloromethyl ketone (TPCK) to inactivate any remaining chymotryptic activity. It is modified by acetylation of the ε-amino groups of lysine residues to prevent autolysis. Trypsin-ultra (TPCKtreated) cleaves at Lys-Pro and Arg-Pro bonds at a much slower rate than other amino acid residues (2).
Digestion with Trypsin-ultra results in a high number of proteins identified, and sequence coverages as high as 90%
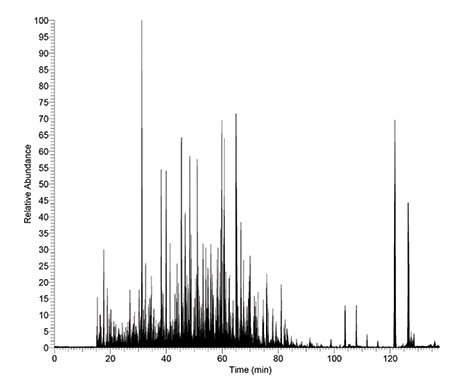
A 3-hour Trypsin-ultra digest of Pyrococcus furiosus using a 1:50 enzyme:substrate ratio, followed by ESI-MS, resulted in 418 proteins identified with individual protein sequence coverage as high as 90%. Product Source
Isolated from bovine (Bos taurus) pancreas.Reconstitution
Trypsin-ultra, Mass Spectrometry Grade should be reconstituted by the addition of 20–200 μl of high purity water. Rapid autolysis is a function of enzyme concentration.- This product is related to the following categories:
- Proteases Products,
- Proteome Analysis Products
- This product can be used in the following applications:
- Glycomics and glycoproteomics,
- Protein Digestion
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.