SHuffle® T7 Competent E. coli
NEB recommends using SHuffle Express strains for best performance.
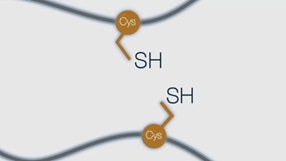
Chemically competent E. coli K12 cells suitable for T7 protein expression with enhanced capacity to correctly fold proteins with multiple disulfide bonds in the cytoplasm.
- T7 expression
- Engineered E. coli K12 to promote disulfide bond formation in the cytoplasm
- Resistant to phage T1 (fhuA2)
- Free of animal products
- NEB does not add a dry ice surcharge to our competent cell shipments
Featured Video
-
Product Information
Chemically competent E. coli K12 cells engineered to form proteins containing disulfide bonds in the cytoplasm. Suitable for T7 promoter driven protein expression. Highlights
- Engineered E. coli K12 to promote disulfide bond formation in the cytoplasm
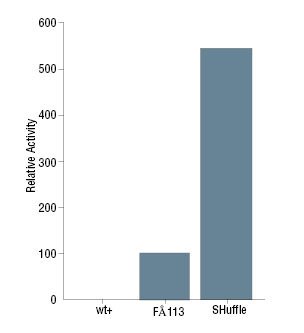
- Constitutively expresses a chromosomal copy of the disufide bond isomerase DsbC
- DsbC promotes the correction of mis-oxidized proteins into their correct form (1,3)
- The cytoplasmic DsbC is also a chaperone that can assist in the folding of proteins that do not require disulfide bonds (4)
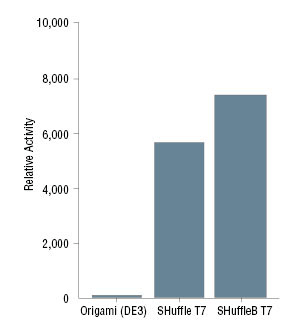
- Expresses a chromosomal copy of T7 RNAP
- Tight control of expression by lacIq allows potentially toxic genes to be cloned
- Resistance to phage T1 (fhuA2), Nit, Str, Spec
Transformation Efficiency
1 x 106 cfu/µg pUC19 DNAGenotype
F´ lac, pro, lacIq / Δ(ara-leu)7697 araD139 fhuA2 lacZ::T7 gene1 Δ(phoA)PvuII phoR ahpC* galE (or U) galK λatt::pNEB3-r1-cDsbC (SpecR, lacIq) ΔtrxB rpsL150(StrR) Δgor Δ(malF)3- This product is related to the following categories:
- SHuffle Expression,
- E. coli Protein Expression Strains Products,
- Protein Expression Products, E. coli Expression Strains Products
- This product can be used in the following applications:
- T7 Expression,
- Expression of Difficult Proteins,
- Disulfide-bonded Protein Expression,
- Protein Expression in E. Coli, Protein Expression
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.