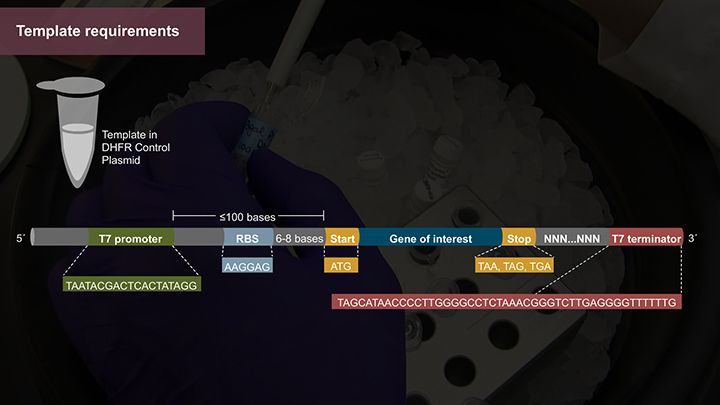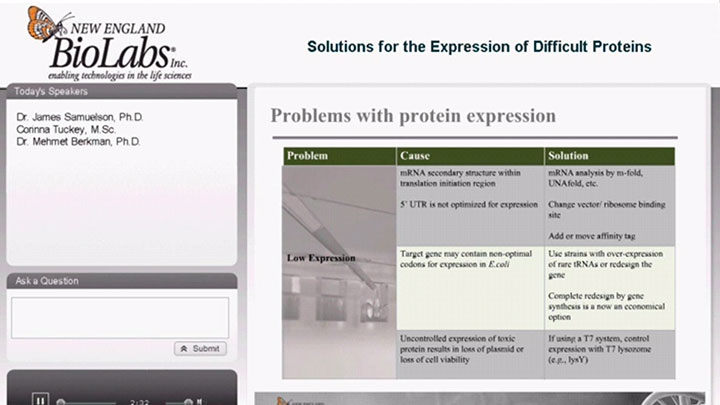
Protein Expression
Choose Type:
- Cell-Free Protein Expression
- NEBExpress® Cell-free E. coli Protein Synthesis System
- PURExpress
- Protein Expression in Yeast
- Expression of Difficult Proteins
- Disulfide-bonded Protein Expression
- Membrane Protein Expression
- Toxic Protein Expression
- Target Protein Insolubility
- Protein Expression in E. Coli
- T7 Expression
- Non-T7 Expression
- Protein Expression Using BL21(DE3) (C2527)
- Protein Expression with T7 Express strains
- Expression Using SHuffle®
- Protein Synthesis Reaction using PURExpress (E6800)
- Western Analysis (E8023)
- Transformation Protocol (C2530)
- Protocol for Protein Expression Using BL21 (C2530)
- 5 Minute Transformation Protocol (C2530)
- Expression Using SHuffle (C3027)
- Use SNAP-Capture Pull Down Resin (S9144)
- Electroporation Protocol (C2986)
- Expression Using SHuffle (C3026)
- Transformation of SHuffle® Competent Cell Strains
- E coli Lemo21 DE3 A T7 RNA Polymerase-based protein overexpression platform for routine and difficult targets
- Co-expression of Multiple Proteins in Kluyveromyces lactis
- Protein Expression with T7 Express Strains
- Use of the PURExpress® in vitro Protein Synthesis Kit, Disulfide Bond Enhancer and SHuffle® Competent E. coli for heterologous in vitro and in vivo cellulase expression.
- Using the PURExpress® In Vitro Protein Synthesis Kit for Heterologous In Vitro Expression and Functional Screening of FMN-dependent Oxidoreductase Variants
- NEBExpress® Cell-free E. coli Protein Synthesis System
- Scaling down to scale up – Miniaturizing cell-free protein synthesis reactions with the Echo 525 Acoustic Liquid Handler
- Accelerating DNA Construction to Protein Expression A Rapid 1-Day Workflow Using NEBridge Golden Gate Assembly
- Simultaneous Fluorescent Labeling of Proteins in Living Cells
-
Avoid Common Obstacles in Protein Expression
Read how to avoid common obstacles in protein expression that prevent interactions with cellular machinery.
-
The Future of Cell-Free Protein Synthesis
Cell-free protein synthesis has the potential to become one of the most important high throughput technologies for functional genomics and proteomics.
-
The Next Generation of Reagents for Sample Preparation
-
Why Choose the K. lactis Protein Expression Kit?
Review the advantages of the K. lactis Protein Expression Kit for rapid, high yield protein expression in yeast.
-
Over 40 years in protein expression and purification – a historical perspective
This article provides an overview of the advances in protein expression and purification methodology over the past 40 years.
- Competent Cell Brochure
- Protein Expression & Purification Brochure
- DNA Sequences and Maps Tool
- Competent Cell Product Comparison
- IMPACT™ Vectors and Applications
- Protein Expression and Purification Selection Chart
- An E.coli lysate-based system for in vitro Protein Synthesis
Feature Articles
Brochures
Web Tools
Selection Tools
Posters
- Agrawal, A., Bisharyan, Y., Papoyan, A, Bednenko, J., Cardarelli, J., Yao, M., Clark, T., Berkmen, M., Ke, N., Colussi, P. (2019) Fusion to Tetrahymena thermophila granule lattice protein 1 confers solubility to sexual stage malaria antigens in Escherichia coli. Protein Expr Purif; 153, 7-17. PubMedID: 30081196, DOI: 10.1016/j.pep.2018.08.001.
- Manta, Bruno; Berkmen, Mehmet; (2019) Disulfide Bond Formation in the Periplasm of Escherichia coli. EcoSal Plus; PubMedID: 30761987, DOI: 10.1128/ecosalplus.ESP-0012-2018.
- Leith, E.M., O'Dell, W.B., Ke, N., McClung, C., Berkmen, M., Bergonzo, C., Brinson, R.G., Kelman, Z (2019) Characterization of the internal translation initiation region in monoclonal antibodies expressed in Escherichia coli J Biol Chem; 294(48), 18046-18056.. PubMedID: 31604819, DOI: 10.1074/jbc.RA119.011008
- Sakhtah, H., Behler, J., Ali-Reynolds, A., Causey, T.B., Vainauskas, S., Taron, C.H. (2019) A novel regulated hybrid promoter that permits autoinduction of heterologous protein expression in Kluyveromyces lactos Appl Environ Microbiol; pii: e00542-19. PubMedID: 31053583
- Reuter, W.H., Masuch, T., Ke, N., Lenon, M., Radzinski, M., Van Loi, V., Ren, G., Riggs, P., Antelmann, H., Reichmann, D., Leichert, L.I., Berkmen, M (2019) Utilizing redox-sensitive GFP fusions to detect in vivo redox changes in a genetically engineered prokaryote Redox Biol; 26, 101280. PubMedID: 31450103, DOI: 10.1016/j.redox.2019.101280
- Reddy, P.T., Brinson, R.G., Hoopes, J.T., McClung, C., Ke, N., Kashi, L. (2018) Platform development for expression and purification of stable isotope labeled monoclonal antibodies in Escherichia coli. mAbs MAbs; 10 (7), 992-1002. PubMedID: 30060704, DOI: 10.1080/19420862.2018.1496879
- Ke, Na; Berkmen, Mehmet; Ren, Guoping; (2017) A water-soluble DsbB variant that catalyzes disulfide-bond formation in vivo Nat Chem Biol; 13, 1022-1028. PubMedID: 28628094, DOI: 10.1038/nchembio.2409
- Chuzel, L., Ganatra, M.B., Schermerhorn, K.M., Gardner, A.F., Anton, B.P., Taron, C.H. (2017) Complete genome sequence of Kluyveromyces lactis strain GG799, a common yeast host for heterologous protein expression Genome Announc; 5(30), PubMedID: 28751387
- Anton, B.P., Fomenkov, A., Raleigh, E.A. and Berkmen, M. (2016) Complete Genome Sequence of the Engineered Escherichia coli SHuffle Strains and Their Wild-Type Parents Genome Announc; Mar 31;4(2), PubMedID: 27034504, DOI: 10.1128/genomeA.00230-16.
- Ren, G., Ke, N. and Berkmen, M. (2016) Use of the Shuffle Strains in Production of Proteins. Curr Protoc Protein Sci; Aug 1, 1;85:5.26.1-5.26.21.. PubMedID: 27479507 , DOI: 10.1002/cpps.11.
- Chatelle C, Kraemer S, Ren G, Chmura H, Marechal N, Boyd D, Roggemans C, Ke N, Riggs P, Bardwell J, Berkmen M (2015) Converting a Sulfenic Acid Reductase into a Disulfide Bond Isomerase Antioxid Redox Signal; 26191605. PubMedID: 26191605, DOI: 10.1089/ars.2014.6235
- Robinson, M.-P., Ke, N., Lobstein, J., Peterson, C., Szkodny, A., Mansell, T.J., Tuckey, C., Riggs, P.D., Colussi, P.A., Noren, C.J., Taron, C.H., Delisa, M.P., Berkmen, M. (2015) Efficient expression of full-length antibodies in the cytoplasm of engineered bacteria Nat Commun; (6)8072, PubMedID: 26311203, DOI: 10.1038/ncomms9072.
- Berkmen, M. (2012) Production of disulfide-bonded proteins in Escherichia coli Protein Expr Purif; 240-251. PubMedID: 22085722
- Hemmis, C.W., Berkmen, M., Eser, M.and Schildbach, J.F. (2011) TrbB from conjugative plasmid F is a structurally distinct disulfide isomerase that requires DsbD for redox state maintenance. J Bacteriol; 193(18), 4588-97. PubMedID: 21742866, DOI: 10.1128/JB.00351-11
- Shouldice, S.R., Cho, S.H., Boyd, D., Heras, B., Eser, M., Beckwith, J., Riggs, P., Martin, J.L.and Berkmen, M. (2010) In vivo oxidative protein folding can be facilitated by oxidation-reduction cycling. Mol Microbiol; 75(1), 13-28. PubMedID: 19968787
- Mauris, J.and Evans, T.C., Jr. (2010) A human PMS2 homologue from Aquifex aeolicus stimulates an ATP-dependent DNA helicase. J Biol Chem; 285(15), 11087-11092. PubMedID: 20129926
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.