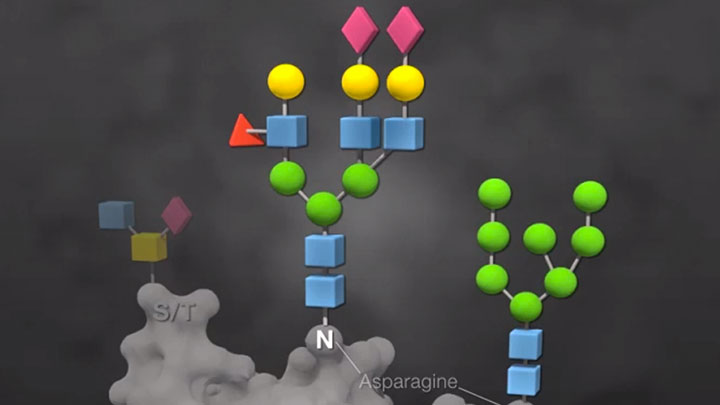
Exoglycosidases
Choose Type:
- Do detergents inhibit exoglycosidases/endoglycosidases?
- What are Glycosidases and their uses?
- Can glycosidases be used in combination for extensive digestion?
- Can glycosidases be used on whole cells?
- Could exoglycosidases change the migration of my protein?
- How are exoglycosidases removed from a reaction?
- How specific are NEB exoglycosidases?
- Endo-α-N-Acetylgalactosaminidase Application Note 1
- Protocol for α1-3,6 Galactosidase (P0731)
- Typical Reaction Conditions for α2-3,6,8 Neuraminidase (P0720)
- Typical Reaction Conditions for α1-2 Fucosidase (P0724)
- Typical Reaction Conditions for β-N-Acetylhexosaminidasef (P0721)
- Typical Reaction Conditions for β1-3 Galactosidase (P0726)
- Typical Reaction Conditions for α1-6 Mannosidase (P0727)
- Typical Reaction Conditions for α1-2,3 Mannosidase (P0729)
- Typical Reaction Conditions for α1-3,6 Galactosidase (P0731)
- Typical Reaction Conditions α-N-Acetylgalactosaminidase (P0734)
- Typical Reaction Conditions for β1-4 Galactosidase S (P0745)
- Typical Reaction Conditions for α2-3,6,8,9 Neuraminidase A (P0722)
- Typical Reaction Conditions for α2-3 Neuraminidase S (P0743)
- Typical Reaction Conditions for β-N-Acetylglucosaminidase S (P0744)
- Typical Reaction Conditions for α1-3, 4, 6 Galactosidase (P0747)
- Typical Reaction Conditions for α1-2, 3, 4, 6 Fucosidase (P0748)
- Typical Reaction Conditions for α1-3, 4 Fucosidase (P0769)
- Typical Reaction Conditions for β1-3,4 Galactosidase Reaction Protocol (P0746)
- Reaction Protocols for Protein Deglycosylation Mix II (P6044)
- Typical Reaction Conditions for a1-2,3,6 Mannosidase (P0768)
- a1-2,4,6 Fucosidase O Digestion of Released Labeled Glycans Protocol
-
The Structure, Function and Importance of Carbohydrates
Read about the structure, function, and importance of Carbohydrates from biology experts at NEB.
- Glycoproteomics Brochure
- Detailed Characterization of Several Glycosidase Enzymes
- Glycobiology Unit Conversion Chart
Feature Articles
Brochures
Usage Guidelines
- Vainauskas, S., Kirk, C.H., Petralia, L., Guthrie, E.P., McLeod, E., Bielik, A., Luebbers, A., Foster, J.M., Hokke, C.H., Rudd, P.M., Shi, X., Taron, C.H (2018) A novel broad specificity fucosidase capable of core a1-6 fucose release from N-glycans labeled with urea-linked fluorescent dyes Sci Rep; PubMedID: 29934601, DOI: 1038/s41598-018-27797-0
- Albrecht, S., Vainauskas, S., Stöckmann, H., McManus, C., Taron, C.H. and Rudd, P.M. (2016) Comprehensive Profiling of Glycosphingolipid Glycans Using a Novel Broad Specificity Endoglycoceramidase in a High-Throughput Workflow. Anal Chem; May 3;88(9), 4795-802. Anal Chem.. PubMedID: 27033327
- O'Flaherty, Roisin, Harbison, Aoife M., Hanley, Philip J., Fadda, Elisa, Rudd, Pauline, Taron, Chris (2017) Aminoquinoline fluorescent labels obstruct efficient removal of N-glycan corex J Proteome Res; 16, 4237-4243.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.