NEB® Turbo Competent E. coli (High Efficiency)
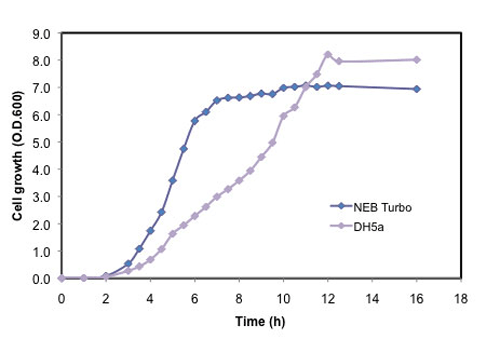
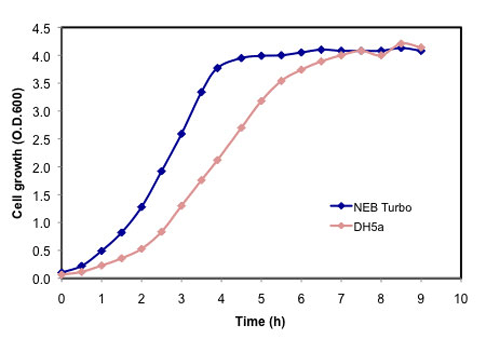
E. coli cells featuring fast colony growth (6.5 hours)
- Tight expression control (lacIq)
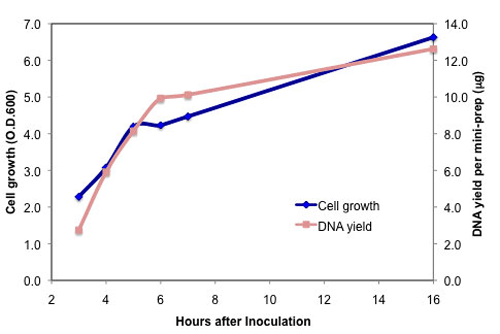
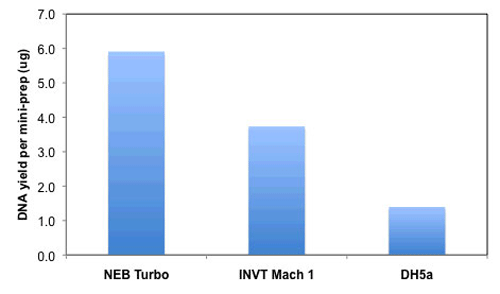
- Isolate DNA after only 4 hours of growth
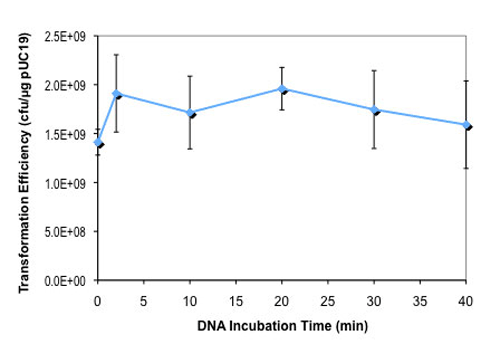
- 5-minute transformation protocol with AmpR plasmids
- No dry ice surcharge on competent cell shipments
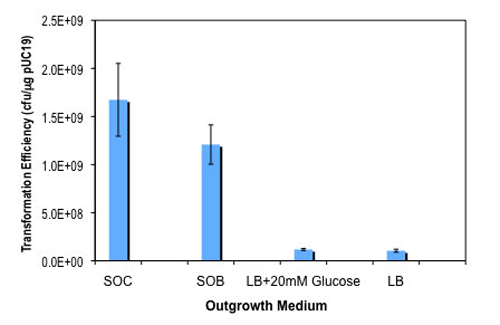
- Outgrowth medium included
- Free of animal products
Featured Video
-
Product Information
Chemically competent E. coli cells suitable for high efficiency transformation and rapid colony growth. Highlights
- Tight control of expression by laclq allows potentially toxic genes to be cloned
- Highest growth rate on agar plates - visible colonies 6.5 hours after transformation
- Activity nonspecific endonuclease I (endA1) eliminated for highest quality plasmid preparations
- Resistance to phage T1 (fhuA2)
- Suitable for blue/white screening by α-complementation of the β-galactosidase gene
- Suitable for 5 minute transformation protocol with AmpR plasmids
- EcoKr-m-, McrBC-
- K12 Strain
- Isolate DNA after 4 hours of growth from a single overnight colony
- Free of animal products
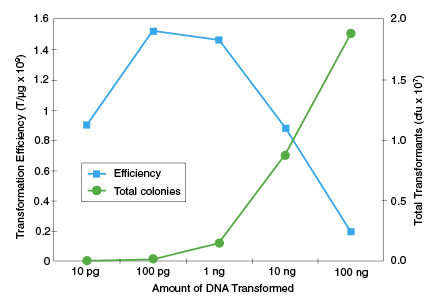
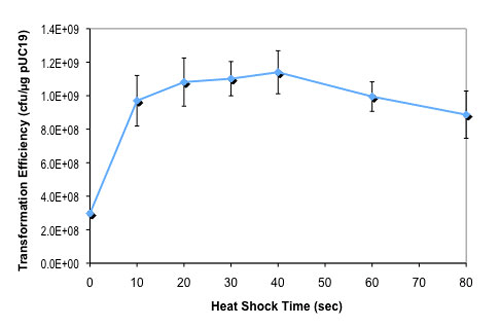
Transformation Efficiency
1 - 3 x 109 cfu/μg pUC19 DNAGenotype
F' proA+B+ lacIq ∆lacZM15 / fhuA2 ∆(lac-proAB) glnV galK16 galE15 R(zgb-210::Tn10)TetS endA1 thi-1 ∆(hsdS-mcrB)5
- This product is related to the following categories:
- Cloning Competent Cell Strains Products
- This product can be used in the following applications:
- High-throughput cloning and automation solutions,
- Transformation,
- Fast Cloning: Accelerate your cloning workflows with reagents from NEB
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.