OneTaq® DNA Polymerases
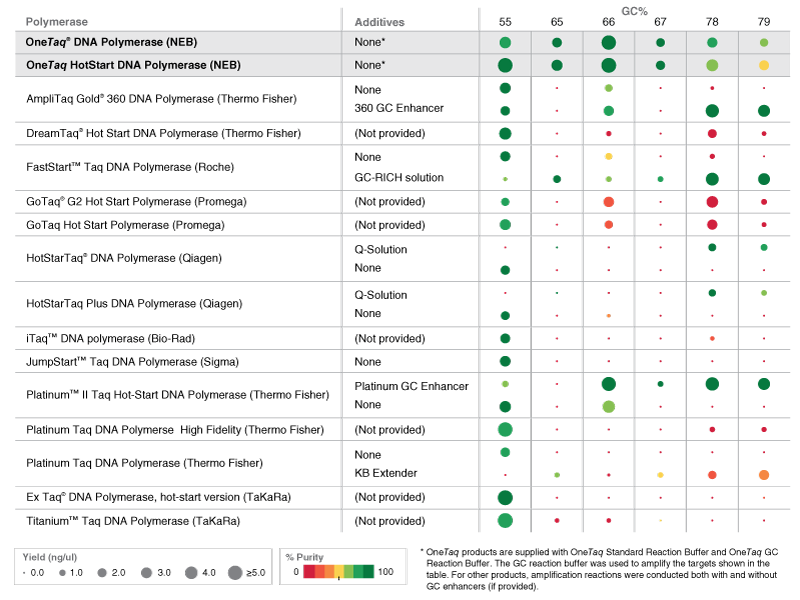
Comparison of OneTaq Products to Other Commercially Available Polymerases
OneTaq Quick-Load 2X Master Mix with Standard Buffer
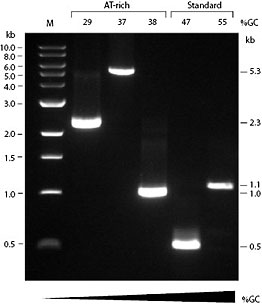
OneTaq Quick-Load 2X Master Mix with Standard Buffer contains an optimized blend of Taq and Deep Vent® DNA Polymerases in a convenient master mix format containing two commonly used tracking dyes for DNA gels. Request a free sample here.
OneTaq Quick-Load DNA Polymerase
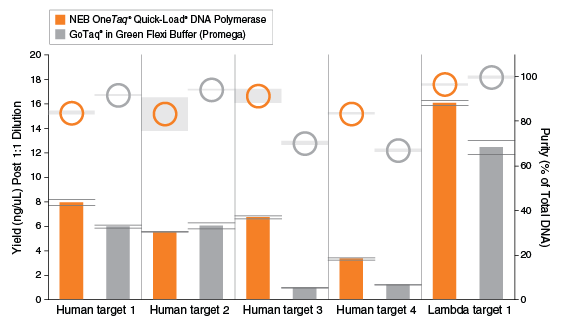
For direct and fast agarose gel loading after routine PCR, such as genotyping and colony PCR, OneTaq Quick-Load DNA Polymerase is available. The product is supplied with a green 5X OneTaq Quick Load Reaction Buffer in addition to the colorless 5X OneTaq Reaction Buffer.
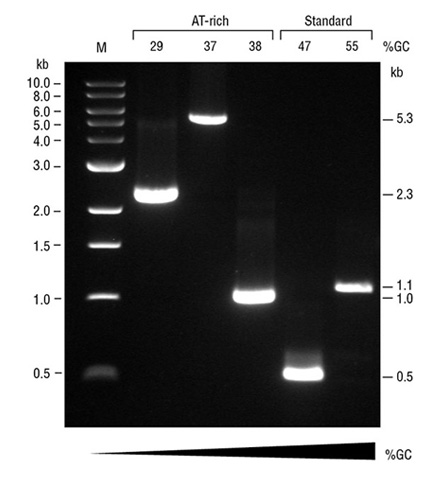
Robust Performance of OneTaq Quick-Load DNA Polymerase
One or more of these products are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc.
AmpliTaq Gold® and FastStart™ are trademarks of Roche Molecular Systems, Inc. DreamTaq® and Platinum™ are trademarks of Thermo Fisher Scientific, Inc. GoTaq® is a registered trademark of Promega Corporation. HotStarTaq® is a registered trademark of Qiagen GmbH, LLC. iTaq™ is a trademark of Bio-Rad Laboratories, Inc. JumpStart™ is a trademark of Sigma-Aldrich Biotechnology LP and Sigma-Aldrich Co. Ex Taq® and Titanium™ are trademarks of Takara Holding, Inc.
Choose Type:
- Protocol for OneTaq 2X Master Mix with GC Buffer (M0483)
- Protocol for OneTaq Hot Start Quick-Load 2X Master Mix with Standard Buffer (M0488)
- OneTaq® Quick-Load® 2X Master Mix with GC Buffer (M0487)
- PCR Protocol for Taq DNA Polymerase
- Protocol for OneTaq Hot Start DNA Polymerase (M0481)
- Protocol for OneTaq® 2X Master Mix with Standard Buffer (M0482)
- Protocol for OneTaq Hot Start Quick-Load 2X Master Mix with GC Buffer
- Protocol for OneTaq Hot Start 2X Master Mix with GC Buffer (M0485)
- Protocol for OneTaq Hot Start 2X Master Mix with Standard Buffer (M0484)
- Guidelines for PCR Optimization with OneTaq® and OneTaq® Hot Start DNA Polymerases
- Protocol for OneTaq® Quick-Load 2X Master Mix with Standard Buffer (M0486)
- PCR Protocol for OneTaq® DNA Polymerase (M0480)
- A-Tailing with Taq Polymerase
- Exceptional performance in endpoint PCR across a wide range of template
- Robust yields with minimal optimization
- Convenient product formats (stand-alone enzyme, master mixes, and Quick-Load® formats)
- Hot start version allows room temperature reaction setup and does not require a separate activation step
- Compatible with standard Taq protocols
- High sensitivity PCR
- High throughput PCR
- Routine PCR
- GC-rich PCR
- AT-rich PCR
- Primer extension
- Colony PCR
- Long PCR (up to ~6 kb genomic)
Template/product specificity: Is RNA or DNA involved? Is the 3´ terminus at a gap, nick or at the end of the template?
Removal of existing nucleotides: Will the nucleotide(s) be removed from the existing polynucleotide chain as part of the protocol? If so, will they be removed from the 5´ or the 3´ end?
Thermal stability: Does the polymerase need to survive incubation at high temperature or is heat inactivation desirable?
Fidelity: Will subsequent sequence analysis or expression depend on the fidelity of the synthesized products?
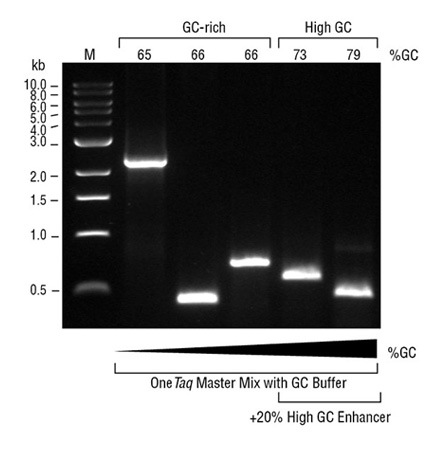
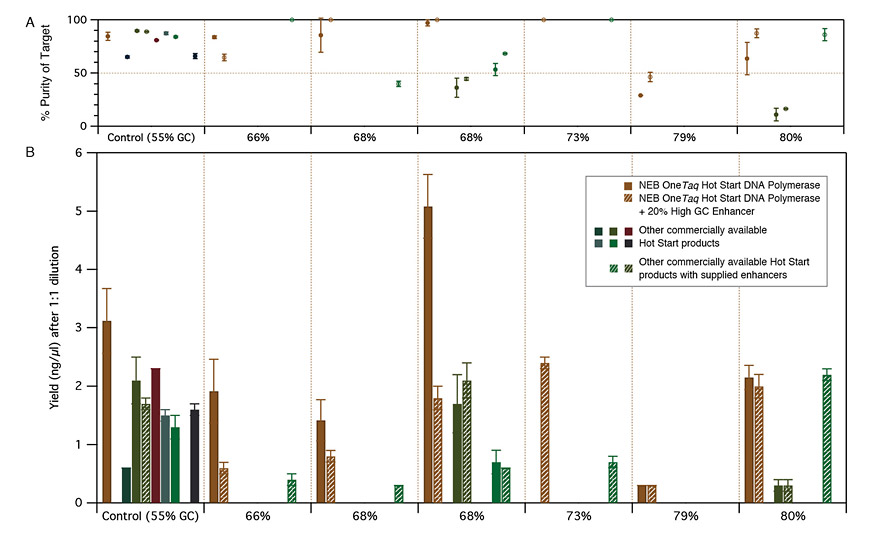
Reactions containing high GC human genomic DNA templates were set up at room temperature. PCR experiments included 30 cycles. Purity (A) and Yield (B) were calculated via microfluidic analysis from triplicate reactions. OneTaq polymerases were used with GC Buffer. Some OneTaq reactions also contained High GC Enhancer (striped bars). Competitor polymerases were cycled according to manufacturer's recommendations and included GC enhancers when supplied (striped bars).
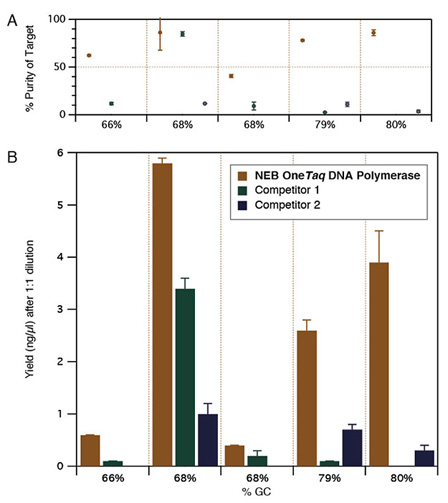
Amplification of a selection of high GC human genomic DNA templates demonstrates OneTaq performance. PCR experiments included 30 amplification cycles. Purity (A) and Yield (B) were calculated via microfluidic analysis from triplicate reactions. Competitor polymerases were cycled according to manufacturer's recommendations.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. The use of this product may require the buyer to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
This product is intended for research purposes only. This product is not intended to be used for therapeutic or diagnostic purposes in humans or animals.