Quick Blunting™ Kit
The Quick Blunting™ Kit is used to convert DNA with incompatible 5´or 3´overhangs to 5´phosphorylated, blunt-ended DNA for efficient blunt-end ligation into DNA cloning vectors.
- Restriction enzyme digested DNA is blunted in less than 30 minutes
- Reactions are performed at room temperature in a ready-to-use mix
- Suitable for restriction enzyme digested DNA, sheared or nebulized DNA or PCR product
Featured Video
-
Product Information
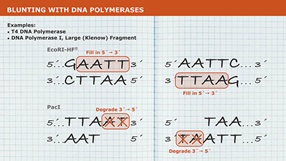
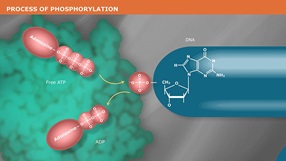
The Quick Blunting Kit is used to convert DNA with incompatible 5´ or 3´ overhangs to 5´ phosphorylated, blunt-ended DNA for efficient blunt-end ligation into DNA cloning vectors. DNA is blunted using T4 DNA Polymerase (NEB #M0203) which has both 3´ → 5´ exonuclease activity and 5´ → 3´ polymerase activity. T4 Polynucleotide Kinase (NEB #M0201) is included in the enzyme mix for phosphorylation of the 5´ ends of blunt-ended DNA for subsequent ligation into a cloning vector. This kit is optimized for blunting up to 5 µg of DNA in a single reaction. To learn more about how to identify what type of overhang you have, visit this video tutorial.
Blunt Enzyme Mix supplied in: 100 mM KCl, 10 mM Tris-HCl(pH 7.4), 0.1 mM EDTA, 1 mM dithiothreitol, 0.1% Triton X-100 and 50% Glycerol.
1X Blunting Buffer:
100 mM Tris-HCl
50 mM NaCl
10 mM MgCl2
0.025% Triton X-100
5 mM dithiothreitol
pH 7.5 at 25°C- This product is related to the following categories:
- DNA Manipulation Products
- This product can be used in the following applications:
- Blunting,
- Phosphorylation (Kinase),
- PCR, Fast Cloning: Accelerate your cloning workflows with reagents from NEB
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.