Home
Applications
Glycobiology & Proteomics
Recombinant Glycoprotein Expression
Glycosaminoglycan Chains (GAGs)
Glycosaminoglycan Chains (GAGs)
Return to Recombinant Glycoprotein ExpressionThe extracellular space of animals has its own class of glycoconjugates. The cell surface and connective matrix of animal tissues have large proteoglycan molecules, which have covalently attached glycosaminoglycan chains (GAGs) that may be either N-linked or O-linked to the core protein.
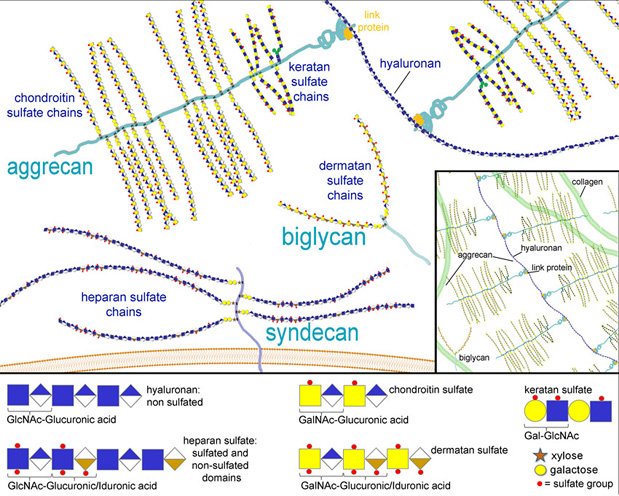
- GAGs are linear polysaccharides that consist of repeating disaccharide units of hexuronic acid linked to a hexosamine.
- GAGs are negatively charged due to numerous sulfate groups.
- GAG biosynthesis occurs without a template and is largely controlled by enzyme and substrate availabilities, resulting in a heterogeneous mixture of glycoforms (1).
- The diverse structural properties of GAGs are important for molecular binding and for tissue morphogenesis (2-4) (Fig. 1).
- Most GAGs are first attached to the core protein and polymerized in the Golgi apparatus, where they are subsequently sulfated.
- This sulfation greatly affects the biological properties of the molecule.
- Hyaluronan, a non-sulfated free polysaccharide is synthesized at the plasma membrane level (5).
References:
- Proteoglycans and Sulfated Glycosaminoglycans. Jeffrey D Esko, Koji Kimata, and Ulf Lindahl. In Essentials of Glycobiology. 2nd edition. Varki A, Cummings RD, Esko JD, et al., editors. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. PMID: 20301236
- Heinegård D. (2009) Int J Exp Pathol. 90(6):575-86. PMID: 19958398
- Sarrazin S, et al. (2011) Cold Spring Harb Perspect Biol. 3(7). pii: a004952. PMID: 21690215
- Haylock-Jacobs S, et al. (2011) Autoimmun Rev. 10(12):766-72. PMID: 21664302
- Maccioni HJ. (2007) J Neurochem.103 Suppl 1:81-90. PMID: 17986143

