Redesigning glycosidase manufacturing quality for pharmaceutical and clinical applications
Introduction
The growing importance of protein glycosylation in both pharmaceutical (1) and clinical science (2) is placing new demands on the quality of reagents used for glycan analysis and manipulation. Over the past 20 years, the list of enzymes and enzymatic specificities available for glycan analysis has steadily grown. Several enzymes have become mainstays in the published literature for applications such as glycan sequencing or N-glycan release from proteins ahead of downstream analytical techniques (Figure 1). Many of these enzymes are transitioning from academic research into workflows that characterize glycans on biotherapeutics and glycan-based clinical tests (4,5). However, many of these enzymes are not being manufactured and quality tested in a manner suitable for use in regulated industries. It is therefore critical that the reagent industry adopt more rigorous quality standards for manufacturing carbohydrate hydrolases for use in clinical and pharmaceutical analytical applications.
Exoglycosidases illustrate the need for manufacturing quality improvements
Exoglycosidases sequentially remove monosaccharides from the non-reducing end of glycans (5). They typically have specificity for a particular type of sugar, its anomeric configuration (α or β), and its position of attachment to an adjacent sugar in an oligosaccharide. They are useful tools because they can modify the composition of glycans either in vitro or in situ (i.e., on the surface of a cell). However, their most common use is in glycan sequencing (6-10). In this application, several exoglycosidases with differing specificities are used in series to decipher the structure of a glycan (Figure 1B; Table 1). Used in conjunction with various analytical methods, like LC-MS or CE-LIF, enzymatic sequencing can provide an orthogonal confirmation of a glycan’s structure. This is particularly applicable to structure characterization of N-glycans derived from biologic drugs to meet with regulatory requirements. 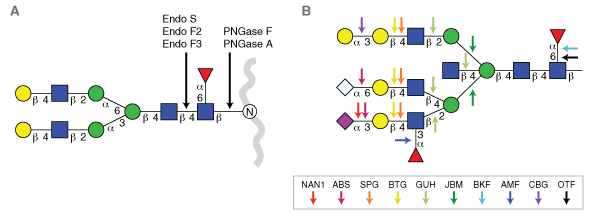
Table 1: Common analytical exoglycosidases
|
COMMON ENZYME ABBREVIATION
|
NEB ENZYME NAME
|
SPECIFICITY
|
ORIGIN
|
SUPPLIER & FORMAT
|
||
|---|---|---|---|---|---|---|
|
NEB
|
PROZYME®
|
SIGMA
|
||||
|
NAN1
|
α2-3 Neu5Ac
|
Streptococcus pneumoniae
|
R | R | R | |
|
ABS
|
α2-3,6,8,9 Neu5Ac/
Neu5Gc |
Arthrobacter ureafaciens
|
R | R | R | |
|
SPG
|
β1-4 galactose
|
Streptococcus pneumoniae
|
R | N | R | |
|
BTG
|
β1-3,4 galactose
|
Bovine (testes)
|
R | R | R | |
|
GUH
|
β N-acetylglucosamine
|
Streptococcus pneumoniae
|
R | N | N | |
|
JBM
|
α1-2,3,6 mannose
|
Jackbeans
|
R | N | N | |
|
BKF
|
α1-2,3,4,6 fucose
|
Bovine (kidney)
|
R | N | N | |
|
AMF
|
α1-3,4 fucose
|
Almondmeal
|
R | – | – | |
|
CBG
|
α1-3,4,6 galactose
|
Coffeebeans
|
R | N | N | |
|
OTF
|
α1-2,4,6 fucose
|
Omnitrophica bacterium
|
R | – | – | |
R = recombinant, N = native source
Exoglycosidases have been used to elucidate glycan structure for over 30 years (6-10). Over this time, several glycosidases have emerged as the field’s preferred enzymes for glycan sequencing due to their desirable specificities (Table 1). However, commercial sources of these enzymes have often lacked supply chain consistency and have had poor overall product quality.
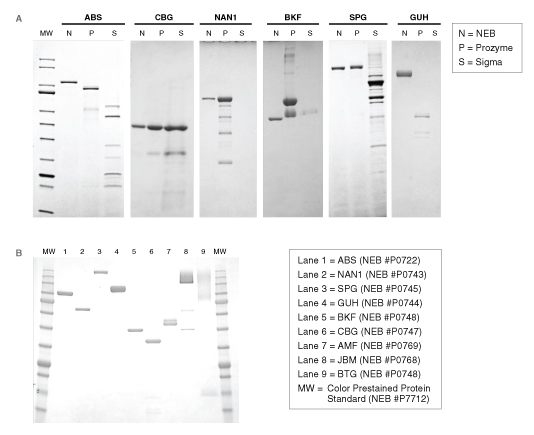
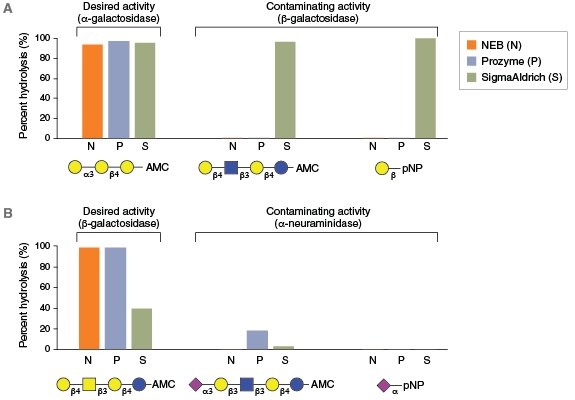
For many glycosidases, quality issues begin from the use of antiquated manufacturing processes. For example, many commercial glycosidases are non-recombinant and are still isolated as native proteins from sources, such as ground plant seed meals (jack beans, almonds, coffee beans) or animal tissues (bovine testes, bovine kidney). These sources are notorious for containing numerous exoglycosidase specificities, contaminating proteases, and often multiple isoforms of the desired enzyme. For example, proteomics analyses of a commercial preparation of native bovine α-fucosidase (BKF) showed that it is a mixture of two different fucosidases (FUCA1 and FUCA2), two similar enzymes with different specificities. The physical similarity and potential interaction between these two enzymes may complicate their ability to be separated in a native purification process. Other examples illustrate that existing commercial supplies of many enzymes vary dramatically in their physical purity (Figure 2) or contain contaminating glycosidase side activities that would severely complicate their use in analytical workflows (Figure 3).
Redesigning glycosidase manufacturing
New England Biolabs® (NEB®) is committed to improving the manufacturing quality of analytical glycosidases with the goal of establishing their routine use in biopharma and clinical diagnostics markets, specifically. NEB's objectives are two-fold:- To improve the manufacturing process design and consistency for analytical glycosidases
- To establish the most rigorous assessment of final product quality in the industry.
To improve overall consistency of its manufacturing process, NEB established new bioprocessing methods for glycosidase production. First, the use of highly variable native enzyme sources, such as seed meals, animal organs, or pathogenic bacteria, was eliminated. Genes were cloned and overexpressed in bacteria or yeast for all NEB analytical glycosidases. For certain enzymes (i.e., jack bean α-mannosidase and almond meal α-fucosidase), this effort involved the discovery of previously unknown plant genes. The use of engineered microbial hosts permitted the design of consistent and scalable fermentation and purification methods. Additionally, NEB has eliminated the direct use of animal-derived components (i.e., in growth medium, chromatography resins or buffers) in each manufacturing process. Finally as part of the product's development, NEB strives to produce three independent production lots for each enzyme to demonstrate process consistency and permit batch-to-batch comparisons.
To improve final product quality, NEB has implemented the industry’s most rigorous program of product quality testing and continual improvement. NEB glycosidases are purified to >95% homogeneity, as assessed by SDS-PAGE and ESI-TOF mass spectrometry. Final products are tested to rule out contaminating endo- and exo-glycosidase activities using 18 independent quality assays (Table 2). Another important advance has been NEB’s use of natural carbohydrate substrates in place of synthetic colorimetric sugar analogs (i.e., p-nitrophenyl (pNP) derivatized sugars) in both quality assurance and unit definition assays. Colorimetric sugar analogs are not hydrolyzed by all exoglycosidases (11). Thus, quality control assays that use such substrates to detect unwanted exoglycosidase contaminants do not fully ensure their absence (see Figure 3B for an example). NEB maintains a large collection of fluorescently-labeled carbohydrate substrates isolated from natural sources, and each glycosidase is subjected to multiple quality tests using these substrates to ensure the absence of unwanted activities in the final product.
Table 2: NEB's extensive quality control assays detect a wide variety of contaminating exoglycosidases
| CONTAMINENT ASSAYED | SUBSTRATE |
|---|---|
| β-Glucosaminidase |  |
| α-Galactosaminidase |  |
| β-Galactosaminidase |  |
| β1,4-Galactosidase & a-Fucosidase |  |
| α-Fucosidase |  |
| β1,3-Galactosidase |  |
| β1,4-Galactosidase |  |
| α1,3-Galactosidase |  |
| α1,6-Galactosidase |  |
| α2,3-Neuraminidase |  |
| α1,3-Mannosidase |  |
| α1,6-Mannosidase |  |
| α1,6-Glucosidase |  |
| Xylosidase |  |
| β-Mannosidase |  |
| Endo F1/H | High mannose mix |
| Endo F2 |  |
| Endo F3 |  |
Finally, important to the needs of biopharma, NEB manufactures under both ISO 9001 and ISO 13485 quality management systems. These international standards validate that all NEB glycosidases are manufactured with the highest level of traceability and quality control. ISO 13485 extends the suitability of NEB glycosidases for use in medical devices. As some molecular diagnostics are regulated as medical devices, glycosidases manufactured to the ISO 13485 standard may have particular relevance in the emerging field of glycan-based molecular diagnostics. Furthermore, NEB is expanding to include cGMP manufacturing capability. This manufacturing option has potential to extend the applicability of NEB glycosidases into glycoengineering where in-process alterations to glycan compositions of biologic drugs or biosimilars could be achieved.
Case study: PNGase F manufacturing
Peptide-N-Glycosidase F (PNGase F) is a broad specificity amidase that liberates high mannose, hybrid and complex N-linked glycans from glycoproteins. It is the most commonly used enzyme for N-glycan removal from glycoproteins. PNGase F digestion is a critical first step in most workflows that permit the analysis of released N-glycans, or deglycosylated proteins or peptides. PNGase F has become increasingly important in the biopharma industry, where defining the structure of N-glycans on therapeutic glycoproteins (e.g., antibodies, fusion proteins) is imperative for defining their performance, quality control, and validating the structural equivalence of biosimilars. Additionally, PNGase F, is increasingly being used in high-throughput analyses of novel glycan-based markers of disease and development of clinical tests. The changing landscape of glycan analysis has placed new demands on PNGase F with respect to its suitability for regulated environments, and for high-throughput workflows.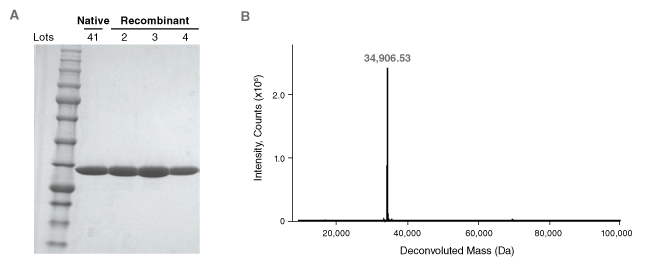
NEB's recombinant PNGase F (Cat #P0708/P0709) is manufactured using the ethos and quality management systems described previously. PNGase F is produced to ≥95% purity, as assessed by SDS-PAGE and intact ESI-MS (Figure 4). The enzyme is subjected to rigorous quality testing to assure the absence of proteases and contaminating exo- and endoglycosidase activities. Over 18 independent quality control assays with labeled glycan substrates is performed on each enzyme lot (Table 2). Finally, three independent production lots are maintained to ensure consistency and reproducibility in the manufacturing process (Figure 4A). The result is the most rigorously quality-tested PNGase F on the market.
Conclusion
The importance of protein glycosylation in pharmaceutical and clinical science has necessitated quality improvements to enzymatic reagents used for glycan analysis in these fields. Many commercial preparations of key analytical glycosidases have significant quality issues and are poorly suited for use in regulated industries. NEB has addressed this need through the development of the industry’s most comprehensive and rigorous approach to glycosidase manufacturing and quality testing. With these quality improvements, NEB glycosidases are poised to address both current and future needs of pharmaceutical and clinical glycan analysis.References
- Zhang, P. et al. (2016) Drug Discov. Today. 21, 740-765.
- Arnold, J.N. et al. (2008) Proteomics. 8, 3284-3293.
- Varki, A. et al. (2015) Glycobiology. 25, 1323-1324.
- Duke, R.M. and Taron, C.H. (2016) Biopharm International. 28, 59-64.
- Guthrie, E.P. and Magnelli, P.E. (2016) Bioprocess Int. 14.
- Kobata, A. (1979) Anal. Biochem. 100, 1–14.
- Rudd, P.M. et al. (1997) Nature. 388, 205-207.
- Prime, S. and Merry, T. (1998) Methods Mol. Biol. 76, 53–69.
- Royle, L., et al. (2006) Methods Mol. Biol. 347, 125–143.
- Mariño, K., et al. (2010) Nat. Chem. Biol. 6, 713-723.
- Wong-Madden, S.T. and Landry D. (1995) Glycobiology. 5, 19-28.
Acknowledgements
The authors thank Andy Bertera and Kiran Gulati for helpful comments.
One or more of these products are covered by patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc. For more information, please email us at gbd@neb.com. The use of these products may require you to obtain additional third party intellectual property rights for certain applications.
Your purchase, acceptance, or payment of and for NEB’s products is pursuant to NEB’s Terms of Sale at www.neb.com/support/terms-of-sale. NEB does not agree to and is not bound by any other terms or conditions, unless those terms and conditions have been expressly agreed to in writing by a duly authorized representative or officer of NEB.
PROZYME® is a registered trademark of Prozyme, Inc.
SIGMA-ALDRICH® is a registered trademark of Sigma-Aldrich Co., LLC.
AGILENT® is a registered trademark of Agilent Technologies, Inc.
© Copyright 2017, New England Biolabs, Inc.; all rights reserved.

