
Improvements in Library Yield and Conversion Rate
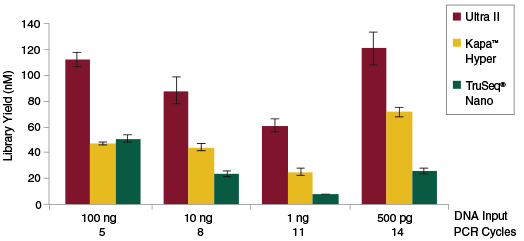
Libraries were prepared from Human NA19240 genomic DNA using the input amounts and numbers of PCR cycles shown. Manufacturers’ recommendations were followed, with the exception that size selection was omitted.
The efficiency of the End Repair, dA-Tailing and Adaptor Ligation steps during library construction can be measured separately from the PCR step by doing qPCR quantitation of adaptor-ligated fragments prior to library amplification. This enables determination of the rate of conversion of input DNA to adaptor-ligated fragments, i.e., sequenceable molecules. Therefore, measuring conversion rates is another way to assess the efficiency of library construction and also provide information on the diversity of the library. Again, NEBNext Ultra II enables substantially higher rates of conversion as compared to other commercially available kits.
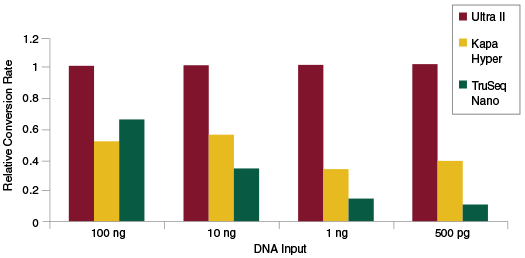
Libraries were prepared from Human NA19240 genomic DNA using the input amounts and library prep kits shown without an amplification step, and following manufacturers’ recommendations. qPCR was used to quantitate adaptor-ligated molecules, and quantitation values were then normalized to the conversion rate for Ultra II. The Ultra II kit produces the highest rate of conversion to adaptor-ligated molecules, for a broad range of input amounts.
In general, it is preferable to use as few PCR cycles as possible to amplify libraries. In addition to reducing workflow time, this also limits the risk of introducing bias during PCR. A consequence of increased efficiency of End Repair, dA-Tailing and Adaptor Ligation is that fewer PCR cycles are required to achieve the library yields necessary for sequencing or other intermediate downstream workflows. For applications such as exome enrichment, very high library yields (1 μg or more) are generally required as input for the enrichment step. If library preparation is inefficient, a large number of PCR cycles may be required to achieve these required yields, especially when the input amount for the original library is low. This can result in production of a library that is not representative of the original sample. View additional data on target enrichment applications.
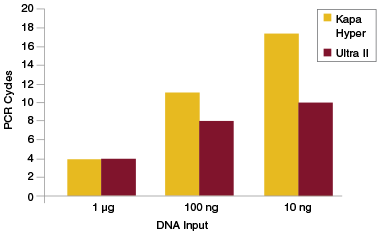
Libraries were prepared from Human NA19240 genomic DNA using NEBNext Ultra II and the input amounts shown. Yields were measured after each PCR cycle and the number of cycles required to generate at least 1 μg of amplified library determined. Cycle numbers for Kapa™ Hyper were obtained from Kapa Biosystems website and plotted alongside the cycle numbers obtained experimentally for Ultra II.
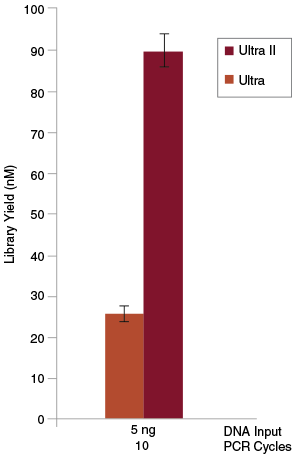
Libraries were prepared from Human NA19240 genomic DNA using the lowest input amount recommended for the original NEBNext Ultra Kit (5 ng) and 10 PCR cycles. Significantly higher yields were achieved with the NEBNext Ultra II Kit than with the original Ultra Kit.



