Preventing COVID-19 Outbreaks through College Campus Surveillance Testing
Posted on Wednesday, May 19, 2021
By
Topic: What is Trending in Science
COVID-19 pooled testing is an efficient way to monitor viral spread within a community, and the loop mediated isothermal amplification (LAMP) assay is a major asset for community monitoring because it's inexpensive, fast, and accurate.
What is surveillance testing and why do we need it?
Surveillance testing involves pooling multiple samples into a single sample for diagnostic testing. This type of testing is especially informative when individuals are infected and asymptomatic, offering a more accurate representation of population infection rates. This allows potential outbreaks to be monitored more carefully, and response plans can be prepared in advance. Based on the level of infection, precautions and mitigation efforts, such as mask-wearing, social distancing and further testing can be employed.
Pooled surveillance testing samples
Surveillance testing samples can be collected several ways. Samples can be pooled manually, as in classroom surveillance testing, where students’ individual nasal swabs are combined into a single tube for analysis. Pooled samples can also be collected from wastewater, which contains viral material shed in the fecal matter from infected individuals. Wastewater surveillance offers an opportunity to passively monitor the infection level at the dormitory, campus, or town level through the use of sewage samples.
Pooled surveillance testing requires that the test employed be sensitive enough to detect low levels of viral RNA within the pooled sample. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is frequently used for pooled sample testing and wastewater surveillance testing because of its sensitivity.
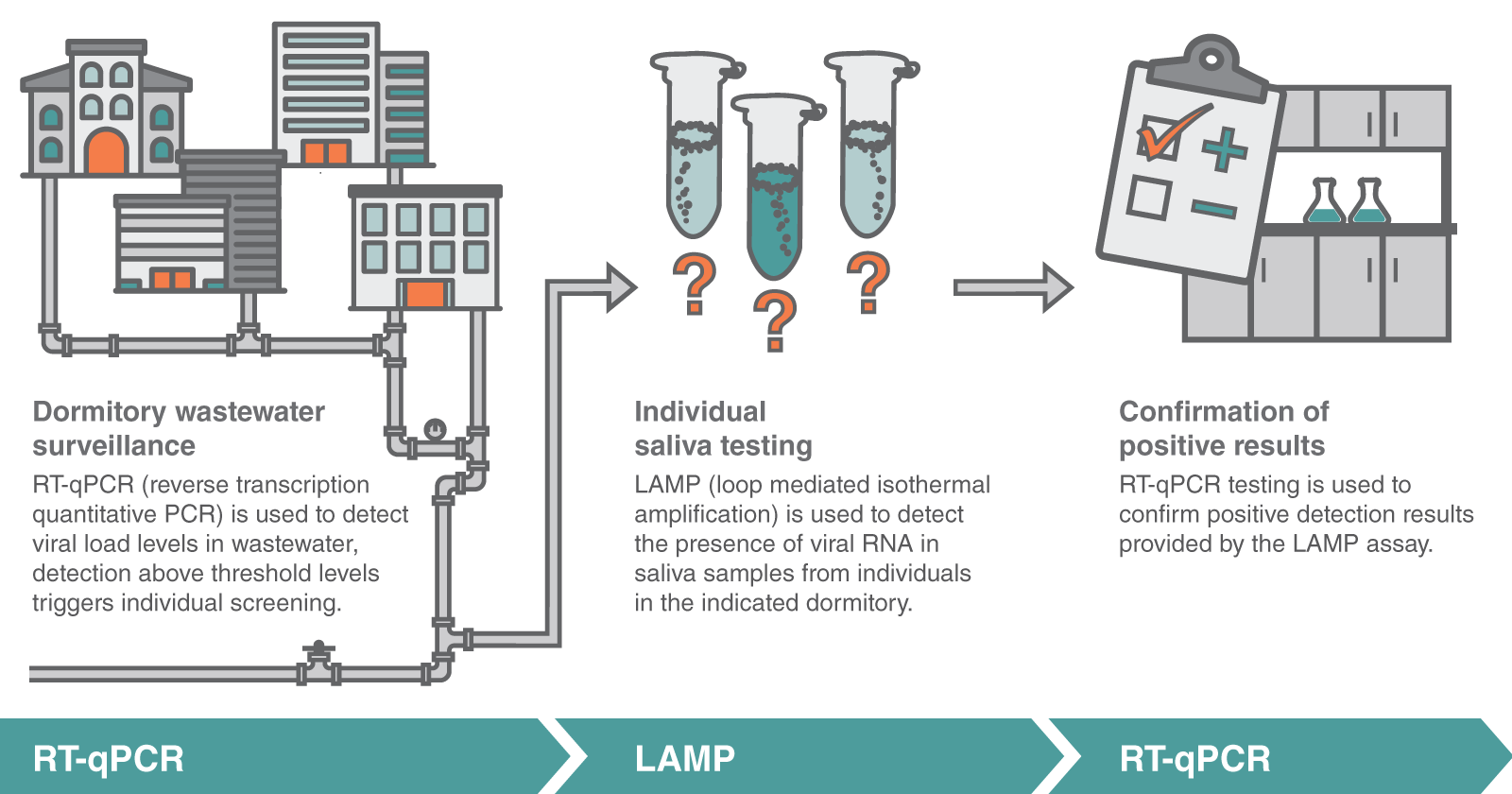
How does campus surveillance testing work?
Professor Todd Camenisch, Chair of the Department of Pharmaceutical Sciences helped build the surveillance testing program at St. John Fisher College and shared some insight with us in a recent podcast. The first stage of their multilayered SARS-CoV-2 surveillance program is wastewater surveillance, beginning with collecting wastewater sludge from multiple dormitory sites twice a week. These samples are tested for SARS-CoV-2, and if the sample signal reaches a certain threshold, it triggers the next level of the testing program – saliva-based testing. Collection bag kits with pre-printed and bar-coded tubes are distributed amongst the residents, who are required to provide saliva samples. These samples are then analyzed using loop-mediated isothermal amplification (LAMP) to detect SARS-CoV-2 in each individual sample.
LAMP utilized 4-6 primers to recognize distinct regions of target DNA for amplification. Synthesis is initiated by a strand-displacing polymerase and 2 carefully designed primers which form “loop” structures to enable subsequent rounds of amplification through extension on the loops and further annealing of primers. The LAMP assay results in long DNA products formed from many repeats of the short target sequence connected by single-stranded loop regions. This target amplification is sufficiently extensive to allow for multiple detection methods, including real-time fluorescence, lateral flow, agarose gel, or colorimetric detection allowing visualization by eye.
Professor Camenisch explained that the method of wastewater collection is also critical, “We switched from doing a manual collection, to where we use autosamplers so we could collect over a 24-hour period, say Sunday evening until the following Monday evening.” It is also recommended that samples are not collected when cleaners or sanitizers are being used in restrooms, as toilet bowl cleaners and other chemicals can affect the viral load present in sewage.
Professor Camenisch’s lab at St. John Fisher College performs the saliva-based testing for campus samples, running about a thousand tests a day. These tests are performed using the LAMP assay, which was chosen for its accuracy and simplicity. When the LAMP assay indicates a positive result, individuals are then tested by RT-qPCR; in these cases, results have been greater than 90% in concordance with the LAMP assay.
Preventing an Outbreak
In late October of 2020, early warning signs indicated that St. John Fisher College was on the verge of a major outbreak, and so based on the wastewater surveillance, LAMP assay and nasopharyngeal confirmatory results, the institution moved to a fully remote campus. Camenisch’s group overlaid their data with available county data and predicted a major upswing in COVID-19 positive cases within Monroe County, which enabled the community to prepare and implement mitigation efforts. “We feel the major advantage of having the LAMP assay is that it did put our college campus community and the destiny in our hands. So, the ability to conduct the detection rapidly and accurately, and provide the data within that same day to the college administration for real-time decision-making was very powerful.” said Professor Camenisch.

NEB will not rent, sell or otherwise transfer your data to a third party for monetary consideration. See our Privacy Policy for details. View our Community Guidelines.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. See www.neb.com/trademarks. The use of these products may require you to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
Don’t miss out on our latest NEBinspired blog releases!
- Sign up to receive our e-newsletter
- Download your favorite feed reader and subscribe to our RSS feed
Be a part of NEBinspired! Submit your idea to have it featured in our blog.



