Oligonucleotide Cleanup Using Monarch® PCR & DNA Cleanup Kit (5 μg) Protocol (NEB #T1030)
Important Update: Beginning in May 2021, we will be gradually transitioning the Monarch DNA Cleanup Binding Buffer to a concentrated format which requires the addition of isopropanol by the user. The protocol below has been updated to reflect this change, but please refer to the instructions provided with your products, as your lot may not be affected.
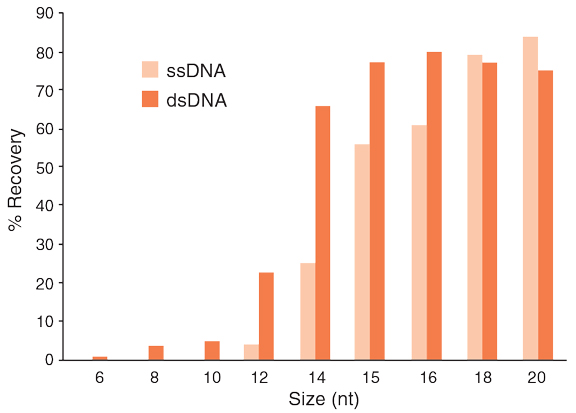
The Monarch PCR & DNA Cleanup Kit protocol can be modified to enable the purification of ssDNA, oligonucleotides, and other small DNA fragments. The following modified protocol utilizes the same columns and bind/wash/elute workflow of the Monarch PCR & DNA Cleanup Kit with > 70% recovery and cleanup of oligonucleotides ≥ 15 bp (dsDNA) or ≥18 nt (ssDNA). The Oligonucleotide Cleanup protocol efficiently removes unincorporated nucleotides, short oligos, dyes, enzymes, and salts from labeling and other enzymatic reactions.
General Guidelines:
Input amount of DNA to be purified should not exceed the binding capacity of the column (5 μg). A starting sample volume of 50 μl is recommended. For smaller samples, nuclease-free water can be used to adjust the volume to the recommended volume range. Centrifugation should be carried out at 16,000 x g in a standard laboratory microcentrifuge at room temperature.Download the Quick Protocol Card.
Before You Begin:
Add isopropanol to Monarch DNA Cleanup Binding Buffer prior to use*:
- For the 50-prep kit, add 14 ml of isopropanol to the DNA Cleanup Binding Buffer.
- For the 250-prep kit, add 63.5 ml of isopropanol to the DNA Cleanup Binding Buffer.
Add ethanol to Monarch DNA Wash Buffer prior to use (4 volumes of ≥ 95% ethanol per volume of Monarch DNA Wash Buffer)
- For 50-prep kit add 20 ml of ethanol to the Monarch DNA Wash Buffer
- For 250-prep kit add 100 ml of ethanol to the Monarch DNA Wash Buffer
Always keep all buffer bottles tightly closed when not in use.
Protocol:
All centrifugation steps should be carried out at 16,000 x g. (~13K RPM in a typical microcentrifuge). This ensures all traces of buffer are eluted at each step.
-
A starting sample volume of 50 μl is recommended. For smaller samples, nuclease-free water can be used to adjust the volume.
- Add 100 μl DNA Cleanup Binding Buffer (ensure that isopropanol has been added, as indicated on the bottle label)* to the 50 μl sample.
- Add 300 μl ethanol (≥ 95%). Mix well by pipetting up and down or flicking the tube. Do not vortex.
- Insert column into collection tube, load sample onto column and close the cap. Spin for 1 minute, then discard flow-through.
 To save time, spin for 30 seconds, instead of 1 minute.
To save time, spin for 30 seconds, instead of 1 minute.
 If using a vacuum manifold** instead of centrifugation, insert the column into the manifold and switch the vacuum on. Allow the solution to pass through the column, then switch the vacuum source off.
If using a vacuum manifold** instead of centrifugation, insert the column into the manifold and switch the vacuum on. Allow the solution to pass through the column, then switch the vacuum source off.
- Re-insert column into collection tube. Add 500 μl DNA Wash Buffer and spin for 1 minute. Discard flow-through.
 If using a vacuum manifold, add 500 μl of DNA Wash Buffer and switch the vacuum on. Allow the solution to pass through the column, then switch the vacuum source off.
If using a vacuum manifold, add 500 μl of DNA Wash Buffer and switch the vacuum on. Allow the solution to pass through the column, then switch the vacuum source off.
- Repeat Step 5 (Optional). This step is recommended for removal of enzymes that may interfere with downstream applications (e.g., Proteinase K).
- Transfer column to a clean 1.5 ml microfuge tube. Use care to ensure that the tip of the column does not come into contact with the flow-through. If in doubt, re-spin for 1 minute to ensure traces of salt and ethanol are not carried over to the next step.
 If using a vacuum manifold: Since vacuum set-ups can vary, a 1 minute centrifugation is recommended prior to elution to ensure that no traces of salt or ethanol are carried over to the next step.
If using a vacuum manifold: Since vacuum set-ups can vary, a 1 minute centrifugation is recommended prior to elution to ensure that no traces of salt or ethanol are carried over to the next step.
- Add ≥ 6 μl of DNA Elution Buffer to the center of the matrix. Wait for 1 minute, then spin for 1 minute to elute the DNA.
Typical elution volumes are 6–20 μl. Nuclease-free water (pH 7–8.5) can also be used to elute the DNA. Yield may slightly increase if a larger volume of DNA Elution Buffer is used, but the DNA will be less concentrated.
Care should be used to ensure the elution buffer is delivered onto the matrix and not the wall of the column to maximize elution efficiency.
 To save time, spin for 30 seconds, instead of 1 minute.
To save time, spin for 30 seconds, instead of 1 minute.
*Beginning in April 2021, the DNA Cleanup Binding Buffer will be changed to a concentrated format which requires the addition of isopropanol by the user. Please refer to the instructions inside of the product that you receive.
**Make sure to follow the manifold manufacturer's instructions to set-up the manifold and connect it properly to a vacuum source.

