Disulfide bond formation in the periplasm of E. coli (3 of 4)
Script
In the periplasmic space of E. coli, protein enter the periplasm in an unfolded state, where an oxidized DsbA, shown here in blue, engages with the reduced cysteines of the substrate protein.
This results in the formation of a disulfide bond, and the reduction of DsbA, shown here in red.
The oxidation/reduction cycle of DsbA, which occurs as the protein enters the periplasm, is iterative and continues until the protein is fully oxidized.
Here, we take a closer look at the mechanism of disulfide bond formation.
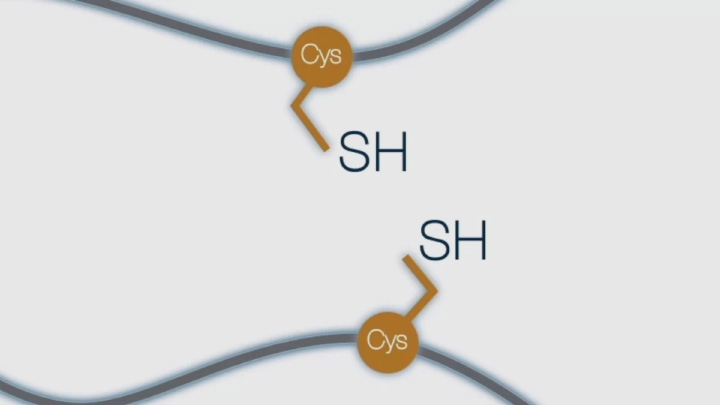
Two cysteines with reduced thiol side chains are “attacked” by an oxidized DsbA, resulting in a transient covalent bond that promotes electron exchange between the substrate protein and DsbA.
This step results in the oxidation of the cysteine thiols of the substrate protein, resulting in the formation of a disulfide bond… and the subsequent reduction of DsbA.
The reduced DsbA is regenerated back to its active, oxidized state, by the integral cell membrane proteins, DsbB. The second periplasmic domain of DsbB oxidizes DsbA and, in turn, it becomes reduced.
The first periplasmic domain then re-oxidizes the second domain and transfers the electrons to quinones present in the membrane. The reduced quinones transfer the electrons to terminal electron acceptors.
Related Videos
-
What is a Disulfide Bond (1 of 4) -
Disulfide bond formation is compartmentalized (2 of 4) -
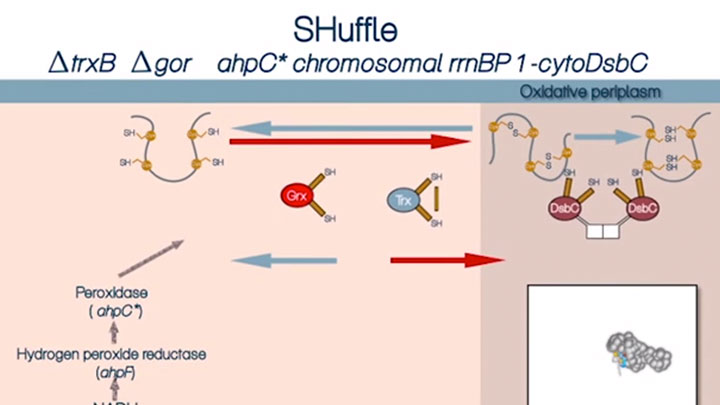
Disulfide bond formation in the cytoplasm of SHUFFLE® cells (4 of 4)

