Insert Considerations When Using NEBridge® Golden Gate Assembly Kit (BsaI-HFv2) (NEB #E1601)
Historically, Golden Gate inserts were precloned into plasmid constructs having flanking BsaI restriction sites to generate the appropriate 4 base overhang sequences that guide the assembly. However, the use of amplicon inserts without precloning also supports efficient assembly levels and saves time. See below for specific recommendations for precloned inserts, and amplicon inserts for single insert cloning and multiple insert assembly:
A. Precloned Inserts: Precloning is always an option, and is recommended for inserts/modules < 250="" bp="" or=""> 3 kb, or those containing repetitive elements that might accumulate errors during PCR amplification to produce amplicon inserts. The pMiniT 2.0 vector backbone used in the NEB PCR Cloning Kit (NEB #E1202/#E1203) is an excellent cloning option as the pMiniT 2.0 Vector backbone has no BsaI sites. Note that all sequences that will be part of the assembly must be flanked by correctly oriented BsaI restriction sites, facing towards the insert on the top and bottom strands.
B. Amplicon Inserts: The 5´ flanking bases and BsaI restriction enzyme recognition site are introduced through PCR primer design upstream and downstream of sequences to be assembled. In all cases, the 2:1 insert:vector (pGGA, 2,174 bp) ratio is suggested to achieve assembly efficiencies similar to that with precloned inserts. For molar calculations we recommend using the NEBioCalculator® (nebiocalculator.neb.com).
(a) Single Insert Cloning/Assembly Primer Design:
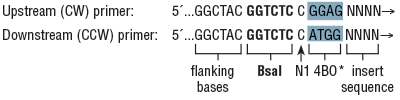
*4 base overhang to allow annealing/ligating into pGGA vector backbone in CW orientation; for CCW assembly orientation, switch the 4 base overhang sequences in the 2 primers.
Single insert amplicons can be used directly from PCR without purification if a specific amplicon is evident upon analysis of part of the reaction by gel electrophoresis. Use the amounts approximating a 2:1 insert:vector ratio and never more than 1 µl to minimize carryover of PCR components that can interfere with assembly. If specificity is not evident, or the concentration is too low, purify amplicon inserts with a spin column. [Monarch® DNA Gel Extraction Kit (NEB #T1020) or the Monarch PCR & DNA Cleanup Kit (5 µg) (NEB #T1030)]. Use of these kits will result in purified, higher concentration DNA due to smaller elution volumes.
(b) Multiple Insert Assembly Primer Design. We recommend using the NEB Golden Gate Assembly Tool (goldengate.neb.com) for the design of PCR primers to ensure the correct 4 base overhangs will be generated and to scan inserts for internal BsaI sites. Purify all amplicon inserts with a spin column protocol .

