Whole-Cell PCR Protocol
Introduction
To facilitate screening of large numbers of transformants, set up PCR reactions using freshly grown cells as a source of chromosomal DNA template.
Protocol
- Transform K. lactis GG799 Competent Cells with a SacII (or BstXI) linearized pKLAC1-based expression construct.
- Streak or patch 10-20 colonies onto fresh YCB Agar Medium plates containing 5 mM acetamide (1.0 cm2 patches are recommended).
- Incubate at 30°C for 1-2 days.
- Immediately transfer the thawed cell stock to the flask containing the equilibrated growth media.
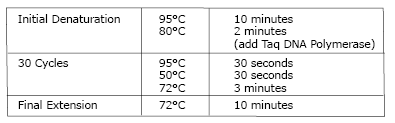
- Use 15 µl of lyticase-treated cells as template in a 100 µl PCR reaction containing either Integration Primers 1 and 2 (to detect proper pKLAC1 integration) or Integration Primers 2 and 3 (to detect multiple integration). Mix all reaction tubes containing cells by vortexing. The following conditions are recommended for the PCR reaction. Note that the Taq DNA Polymerase (NEB #M0267 or NEB #M0273) is added during the 80°C incubation.
- Analyze 10 µl of each amplification reaction on a 1% agarose gel.
- Test strains harboring a properly integrated expression fragment for secretion of the protein of interest.

