TransPass D1 Protocol 1: Transfection in the presence of serum
Introduction
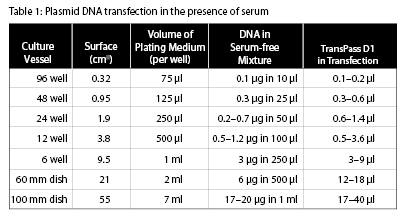
The amounts below are given for a 12-well plate format. Use Table 1 to adjust the reagent volumes for other plate sizes.
Protocol
- Plate cells (in complete growth medium containing 5-10% serum and no antibiotics/antimycotics) at an appropriate density so that they will reach 70-80% confluence at the time of transfection.
- Mix 0.5-1.2 µg plasmid DNA in 100 µl serum-free medium.
- Briefly vortex the tube of TransPass D1 Transfection Reagent and pipet 0.5-3.2 µl to the DNA/medium mix from step 2. Mix gently by flicking the tube.
- Incubate at room temperature for 20-30 minutes to form the transfection complex.
- Add the transfection complex mixture to cells. Rock the plate gently in order to evenly disperse the complex mixture.
- Return the plate to the incubator and incubate 24-72 hours before assaying.
- Replace medium as needed to maintain healthy cells.