Enhancing Molecular Diagnostics in Regulated Markets with Lyophilized Assays
by Jenny Loeb, M.Sc., and Nathan Tanner, Ph.D., New England Biolabs®, & Martin Lee, Ph.D., NEB Lyophilization Sciences™
Molecular diagnostic assays are developed to identify and quantitate specific DNA, RNA, or protein biomarkers found within samples of interest. In many regions, molecular diagnostic testing may only be performed by procuring assays subject to regulatory approval, or with validated tests and workflows in approved laboratories.
Historically, molecular diagnostic assays have utilized cold storage reagents, which require controlled temperature cabinets and dry or wet ice shipment. However, recently, there has been a dramatic increase in the need for molecular diagnostic testing in near-patient and point-of-care settings, and ambient-stored reagents are a key factor in enabling this testing. This requirement has driven the markets towards lyophilized reagents that can be stored and shipped at ambient temperatures.
Obtaining regulatory approval for human in vitro diagnostic tests requires significantly greater time and consideration than developing Research Use Only (RUO) assays typically used in general research laboratories. Nonetheless, it is important to highlight that the developmental steps, performance and safety information, and the data needed for review by regulatory authorities remain consistent regardless of whether wet or lyophilized reagents are utilized.
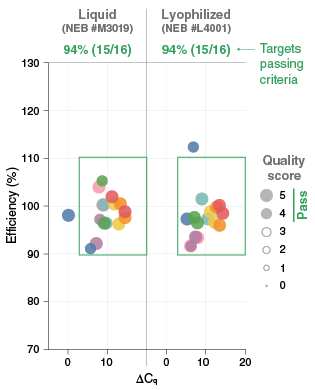
Transitioning an assay that utilizes wet reagents to an assay in a dry form is not as simple as just lyophilizing the wet reagents. Commonly-used liquid reagent formulations contain components such as glycerol or co-solvents that either do not allow for lyophilization or impact the stability and performance of a lyophilized version of the formulation. Many of these liquid-form components help with enzyme stabilization and assay sensitivity, which makes removing components problematic. However, many alternatives to these materials can restore assay performance, and these components can be identified through substitution testing. An additional consideration for lyophilization is the need for excipients and cryoprotectants that protect the macromolecules during freezing and provide for the preservation of the dried material. While it is critical to ensure optimal assay performance when excipients are included in the formulation, it should be noted that they may also act as alternative assay enhancers (Figure 1).
Just like when building a diagnostic assay with liquid reagents, the developer of a lyophilized assay must consider who their end user will be; how the assay will be incorporated into the laboratory’s workflow; which instruments are critical for compatibility; and what quality and regulatory requirements will govern its use. When working with multiple vendors and components to build the final assay, it is important to understand any reconfiguration the developers may undertake when the lyophilized assay is supplied as a sub-component of a workflow or device. Understanding the requirements for handling, transfer and repacking will help the development team make the appropriate product. Vendors specializing in lyophilization can guide development teams on design-for-manufacturing (DFM) options. Ensuring efficient communication of this information at the start of the development process will help the team stay on track as the assay developer completes the product realization process.
Packaging
Product aging for a lyophilized material begins when it is exposed to the atmosphere. Part of the product manufacturing process should be to verify the stability of the assay if it is exposed to the atmosphere. While not an obvious consideration, selecting the appropriate primary packaging format can also impact stability and performance of a lyophilized product over time. The optimal format and composition of the packaging is dependent on the workflow compatibility within the required integrated instruments. Most workflows and integrated instruments will have an assay housed in a molded plastic vessel designed to work with the associated instrumentation.
Many plastics are not resistant to moisture and oxygen ingress, and as a result, the lyophilized assay within the vessel will start to absorb moisture and oxygen even when sealed in this type of container. Therefore, secondary packaging that is resistant to both moisture and oxygen ingress is often used. Secondary packaging includes metalized polymer bags that may be zip-lock and/or heat sealed. The secondary packaging is often sealed under an inert atmosphere, such as nitrogen gas, and may include a desiccant material to adsorb residual moisture. While this packaging should be designed to minimize the reagents’ exposure to both moisture and air, in many cases it should also be designed to minimize exposure to light. Many fluorogenic assay components used in real-time analysis are light sensitive and, if not protected, can degrade over time, reducing assay performance
Optimizing lyophilization conditions and process
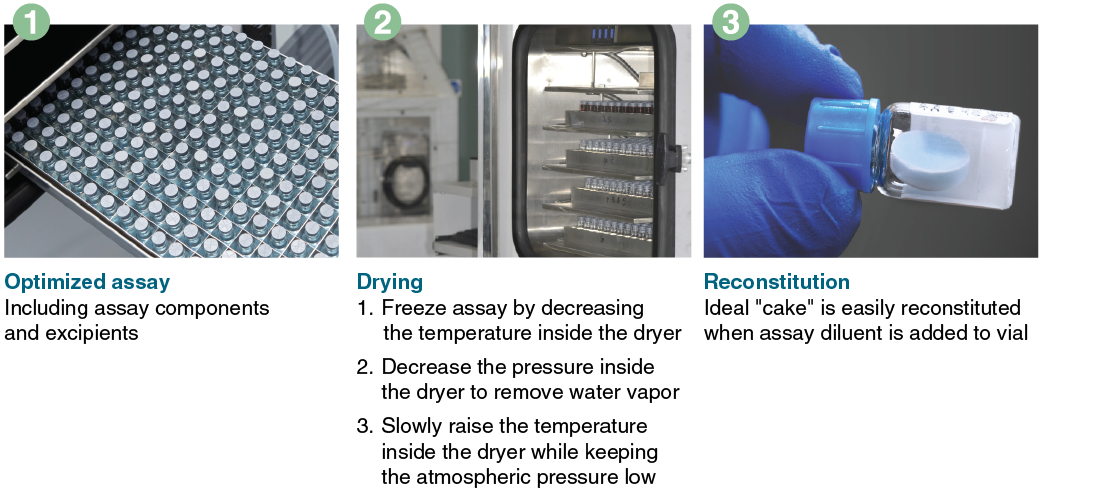
As with optimizing the formulation of the assay components for performance, there is also a requirement to optimize the lyophilization process conditions (Figure 2). Lyophilization is conducted through a controlled process of temperature and pressure changes to facilitate drying. The overall time of each step and the total process will depend upon the formulation of the wet reagents, reagent volume, and container geometry. Poorly optimized processes can lead to incomplete drying and reagent collapse (i.e., the resulting reagent cake losing its intended structure). Products of incomplete drying and collapse can exhibit poor stability and other issues, such as poor dissolution characteristics and inconsistent assay performance. Just as it is possible to lyophilize an assay incompletely, it is also possible to lyophilize “too much” and make an assay that is too dry, resulting in poor enzyme(s) performance.
Process Validation
The ISO 13485 standard requires that the processes used to make a product are robust. In some instances, where measurements may not be easily applied, process validation shall be required. For example, simple temperature and pressure measurements in a freeze dryer will not provide sufficient information that the drying process is robust.
Process validation involves testing using predetermined (outcome) values to demonstrate the process meets its requirements. Such processes include mixing reagents, dispensing methods, lyophilization, and packing processes such as the application of heat seals to plates and bag closures. For lyophilization, the drying process requires sampling plans for specific dryers to ensure that the process performs well at all locations on all shelves within the freeze dryer cabinet. These processes complement product validation to ensure that the test is robust in manufacturing, storage and intended use, and reduces any risks of the test affecting patient safety.
Product Validation and Verification
Multiple parameters must be demonstrated to show that an assay meets its intended use. Some parameters are assay reproducibility, analytical sensitivity and specificity, repeatability, false positive and false negative rates. These are carried out as part of pre-analytical studies, usually prior to clinical testing. For infectious disease testing, this will also include inclusivity and exclusivity testing. Inclusivity testing determines the percentage of target samples that correctly determines that a sample is positive. In contrast, exclusivity testing
determines the percentage of non-target sample that correctly determines that a sample is negative. The testing of interfering substances on the test will also be carried out.
Clinical assay reproducibility looks at how consistent assay results are when performed at multiple trial sites using pre-defined population sample sets. Clinical trials typically determine and validate the products’ clinical sensitivity, specificity, and the test’s positive and negative predictive value.
Technological advances over the past few years have made it possible to shift some molecular diagnostic tests away from only being performed in specialized laboratories to point of care testing locations, including in-home testing. One of the advancements
that has permitted this shift is the ability to create lyophilized assays that are stable at ambient temperatures and perform equivalently to traditional assays making point-of-care testing more accessible across the globe.
View a PDF of this feature article
To learn more about lyophilization services available through NEB Lyophilization Sciences, visit www.neb.com/lyosciences.
To learn more about how we can work with you to develop your molecular diagnostic assay, contact us at custom@neb.com.

