Size Selection of Adaptor-ligated DNA (E7420)
 For libraries with different size fragment inserts, refer to Table 1 for the
appropriate volume of beads to be added. The size selection protocol is
based on a starting volume of 100 μl. The protocol below is for libraries
with a 300–450 bp insert size.
For libraries with different size fragment inserts, refer to Table 1 for the
appropriate volume of beads to be added. The size selection protocol is
based on a starting volume of 100 μl. The protocol below is for libraries
with a 300–450 bp insert size.
- Vortex AMPure XP beads to resuspend.
- Adjust the final volume after ligation by adding nuclease free water for a
100 μl total volume.
- Add 40 μl of resuspended AMPure XP beads to the 100 μl ligation
reaction. Mix well by pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Quickly spin the tube and place the tube on an appropriate magnetic stand
to separate the beads from the supernatant. After the solution is clear
(about 5 minutes), carefully transfer the supernatant containing your DNA
to a new tube (Caution: do not discard the supernatant). Discard the
beads that contain the unwanted large fragments.
- Add 20 μl resuspended AMPure XP beads to the supernatant, mix well and
incubate for 5 minutes at room temperature.
- Quickly spin the tube and place it on an appropriate magnetic stand to
separate the beads from the supernatant. After the solution is clear (about
5 minutes), carefully remove and discard the supernatant that contains
unwanted DNA. Be careful not to disturb the beads that contain the desired
DNA targets (Caution: do not discard beads).
- Add 200 μl of 80% freshly prepared ethanol to the tube while in the
magnetic stand. Incubate at room temperature for 30 seconds, and then
carefully remove and discard the supernatant.
- Repeat Step 8 twice for a total of three washes.
- Air the dry beads for 10 minutes while the tube is on the magnetic stand
with the lid open.
- Elute the DNA target from the beads into 25 μl of 10 mM Tris-HCl or
0.1 X TE, pH 8.0. Mix well on a vortex mixer or by pipetting up and down.
Quickly spin the tube and place it on a magnetic stand. After the solution is
clear (about 5 minutes), transfer 20 μl to a new PCR tube for amplification.
Note: Be sure not to transfer any beads. Trace amounts of bead carry over may affect the optimal performance of the polymerase used in the NEBNext High-Fidelity 2X PCR Master Mix in the subsequent PCR step
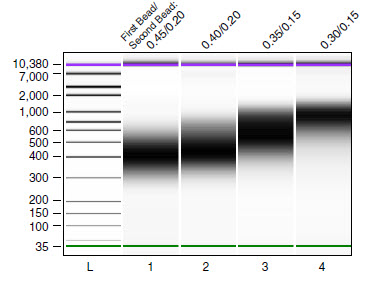
RNA libraries made from Universal Human Reference Total RNA (500 ng) and size selected using different bead/DNA rations as indicated in Table 1. RNA was fragmented at 94°C for 5 minutes.

