
Bringing the International Space Station into the Molecular Age
Posted on Thursday, March 10, 2022
By
Topic: What is Trending in Science
Or is it?
“It’s like a dirty gym”, says one media source, “It is riddled with germs”, says another.
Not so, says Dr. Sarah Wallace – and if anyone knows, she does; Dr. Wallace is a lead in the microbiology lab at NASA’s Johnson Space Center. Her lab is responsible for pre- and in-flight monitoring of the microbes that may be hitching a ride to the International Space Station (ISS) on cargo, in water systems, or more commonly, on the astronauts themselves.
Astronauts from 15 nations have continually inhabited the ISS since November 2000. In other words, in the past 20+ years, there has never been a time when there have not been astronauts – and the millions of microbes that make up the human microbiome – on the space station. With that in mind, you might be surprised to hear that culture-based analysis of the first 29 missions to the ISS showed that it was, in fact, cleaner than the average home!
The primary goal of Dr. Wallace’s lab is identifying and quantifying medically significant pathogens and assessing the risk they pose to the crew. Prior to human occupancy on the ISS, an international consortium established stringent microbial acceptability limits for water, air, surfaces and food. For example, drinking water on the ISS is recycled from humidity condensate, urine and other grey water that undergoes processing. Still, the processed water consumed by the crew is cleaner than what most of us drink on Earth because of the strict microbial and chemical limitations.
But NASA has its sights set on exploration further into space than the ISS and low-Earth orbit, namely back to the Moon and eventually to Mars. Unlike the ISS, the spacecraft involved in these missions will not be regularly replenished with supplies or have the ability to return samples to Earth for analysis. Therefore, Dr. Wallace’s team also creates modified-for-spaceflight molecular workflows to diagnose potential microbial-based threats in near real-time, entirely onboard the spacecraft.
Traditional microbial monitoring on the ISS relies on Earth-bound methods
In the past, the tiniest inhabitants on the ISS, both commensal microorganisms and potential pathogens, have been monitored and identified using culture techniques. These methods have been used since the Apollo missions, and while the Apollo astronauts were not familiar with the more recent term ‘microbiome’, they were, in fact, doing a microbiome assessment of the capsule and crew, both pre- and post-flight. However, there are limitations to this culture-based analysis. Samples collected by the astronauts onboard the ISS need to be transported back to Earth for a complete biochemical and sequence analysis and identification. Additionally, the growth media and conditions used onboard the ISS are favorable to certain microbes, while others that may be present will not grow under these conditions.In recent years, Dr. Wallace and her team have been moving microbial identification on the ISS into the molecular age utilizing techniques such as PCR and nucleic acid sequencing. The ability to identify organisms onboard has enormous implications for extended periods of travel when identification and remediation of potential hazards will need to be dealt with in near real-time. Until recently, this was not possible.
Why monitor for microorganisms in space?
It may seem like an obvious question when posed to a scientific audience, but there are a few reasons, including one or two that you might not have considered:(1) Diagnostic potential and environmental monitoring – when using culture-only techniques to identify microorganisms on the ISS or an infection in a crew member, there are no inflight diagnostic capabilities. The samples are plated on growth media and must be returned to the microbiology laboratory for identification. The crew can assist with this process by sending pictures back to the lab on Earth, where the microbiologists can attempt to visually identify the material, but this amounts to not much more than a guess. Establishing a molecular identification process on the spacecraft would provide the space program with the ability to identify a potential pathogen in near real-time. This can then guide treatment or remediation, taking away the guesswork.
(2) Planetary protection – humans are curious creatures and like to push the limits of exploration. Thankfully, in the time since the first moon landing, awareness has grown around our responsibility to protect these previously uninhabited places. If astronauts can characterize the microbiome of the spacecraft, the potential for contaminating the surface of the Moon or Mars would decrease significantly – we’ve come a long way since waste was left on the Moon 50 years ago! It would also be advantageous to identify biomolecules in the Lunar and Mars environment and, hopefully, not unintentionally bring anything back to Earth.
(3) Applications on Earth – as we push the boundaries of science in space, there are also important implications for here on Earth. While PCR and nucleic acid sequencing are not new technologies, their application in space requires extensive methodology development. The ability to conduct these experiments anywhere, particularly in extreme environments, lends itself to other applications on Earth, for example, understanding the hospital room environment or other point-of-care locations.
(4) Monitoring change – the spaceflight environment has a variety of impacts on living organisms – phenotypic changes and underlying molecular changes that can be difficult to predict. Characterizing these changes is vital to understanding the impact of the space environment on microbial behavior.
What does molecular biology in space look like?
In April 2016, the Genes in Space® program launched a small (palm-sized) thermocycler (miniPCR®) into space and onto the ISS. This advance resulted from a talented high school student’s winning Genes in Space experiment and was the first molecular biology experiment ever done in space.
Soon after, Dr. Wallace’s team sent the MinION®, a very small device that can sequence long stretches of DNA, RNA and modified nucleic acids in real-time, to the ISS. The MinION works by passing a current across an electrically resistant synthetic membrane with embedded protein nanopores. As the nucleic acids move through the pores, they cause a disruption in the current characteristic of a particular molecule, which can be analyzed to determine the sequence of bases.
The miniPCR and MinION together became a great foundation for a portable, remote amplification and sequencing protocol, but deploying it on the space station wasn’t as simple as buying a nucleic acid extraction kit for use on the ISS. Successfully conducting molecular experiments in space has required a gradual workflow expansion. A workflow that may appear straightforward to a molecular biologist in a lab on Earth involves many considerations concerning crew safety, environmental control, and life support systems when conducted in space. For example, as mentioned earlier, water onboard the ISS is recycled, and therefore alcohol, typically used for washing nucleic acids, cannot be used in the sample prep. Additionally, the mechanical lysis of a sample with a traditional bead beater is a no-go, as are most centrifuges. Lab instruments must have critical value due to the extremely limited physical space.
One of the first sequencing experiments in space involved a process requiring only flow cell washing and sample loading. This was a proof-of-concept experiment to show that the MinION could be used in microgravity to sequence samples from various sources. Bacteriophage, E. coli and mouse samples were prepared on Earth and launched frozen in a syringe with a Luer-Lok® tip that allows for leak-free connections – similar to a pipette tip and sized so that it easily fits into the sample port. But this simple methodology gave encouraging results – the astronauts obtained 1000 nucleotide reads, which was directly parallel to what the scientists in the Wallace lab were seeing in identical experiments conducted on the ground.
The ultimate goal was to go from swabbing a surface through to sequencing, all while onboard, and this took years of methodology development. Firstly, using a diluted microbial standard and later taking cells from a contact slide (containing growth media with bacterial colonies), the crew amplified DNA, eliminated primers using an enzymatic clean-up, prepared the DNA libraries, and sequenced. And in the process, they examined every possible MinION metric that they could. While this wasn’t starting from a swab, confidence in this new methodology was building. Using the contact slide, the crew identified two colonies as Staphylococcus hominis and Staphylococcus capitis. When the contact slide returned to Earth, the circled colonies identified in space were sequenced (using traditional Sanger sequencing) and biochemically profiled. They were, in fact, Staphylococcus hominis and Staphylococcus capitis! This was monumentous because it was the first time microbes had been collected, cultured, and identified off Earth - a capability that had not previously existed. You might even say - a giant leap for spaceflight microbiology!

Dr. Peggy Whiston sets up her laboratory bench onboard the ISS as part of the Genes in Space-3 experiment. During this investigation, Dr. Whitson demonstrated the first identifications of microbes collected and cultured off Earth.
Burton, A. S., et al (2020) Genes. PMID: 31936690
They weren’t happy to just stop there. The next hurdle to overcome was associated with sample type; for example, a surface swab typically contains very low biomass – even on Earth it can be challenging to get a good amount of material from a swab. The swab-to-sequencer, culture-independent method was years in development. Hundreds of parameters needed to be tested. It was eventually tested on swab samples collected from surfaces in the ISS. Again, identification based on the molecular approach matched the standard culture-based methods, however with more diversity – more on that, later.
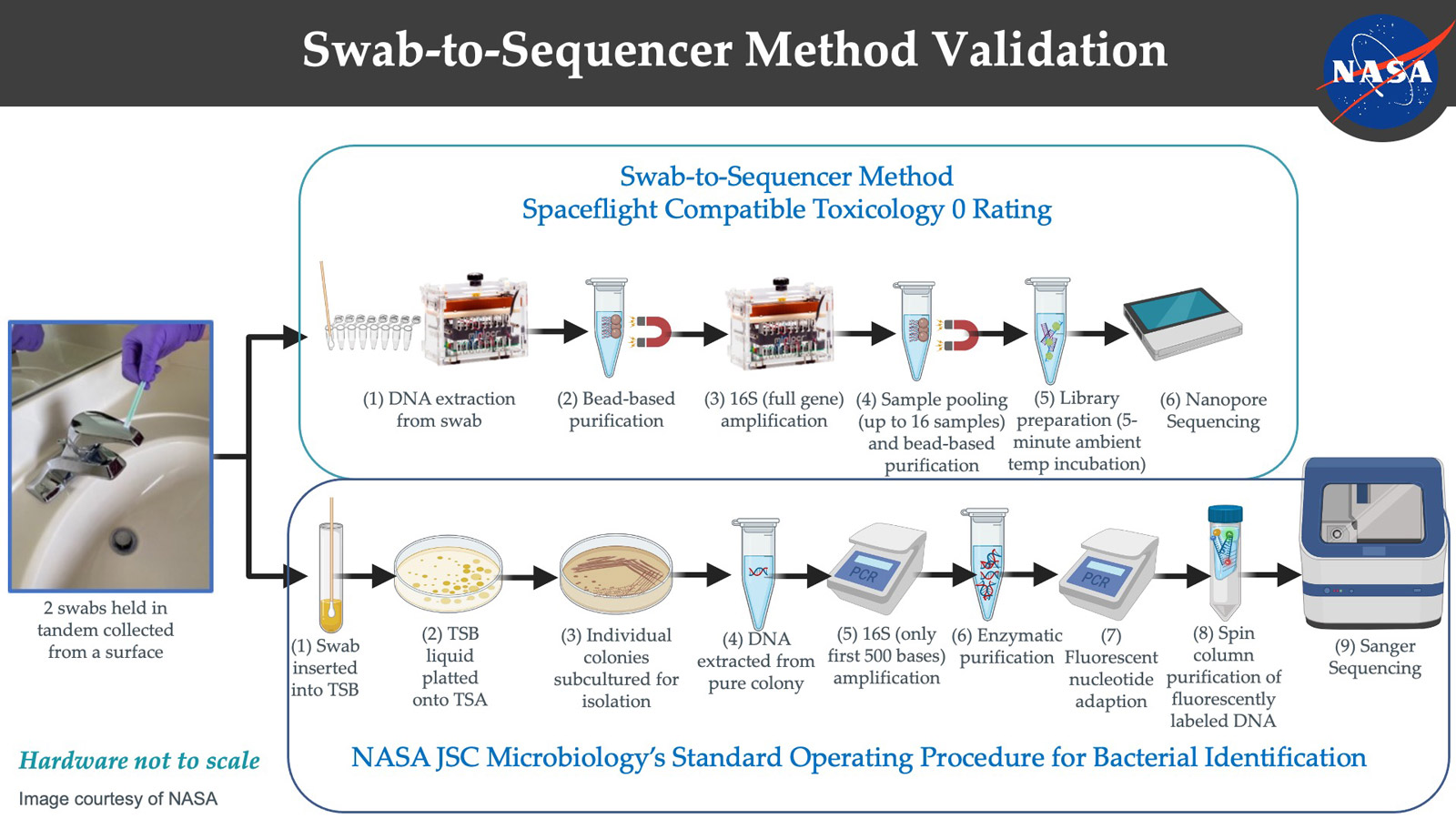
New methodologies were compared to the current culture-based standards to demonstrate the ability to get equivalent information. Two swabs were used in tandem, (top) one for DNA extraction, purification, amplification, sequencing, and data analysis; (bottom) one using traditional culture-based methodology with end identification using Sanger sequencing.

Workflows developed in the Wallace microbiology lab at the Johnson Space Center need to undergo a lot of testing in different environments before being tested on the ISS. Testing is always coupled with comparisons to culture-based methods to validate results. Now, keep in mind that molecular biologists and microbiologists conduct this testing on Earth, but protocols also need to work in the hands of crew members in extreme environments. Microbiologists from the lab host onboard training sessions with the astronauts using pipettes and food coloring. They also modify workflows so the crew does not have to pipet very small volumes where technique is crucial to accuracy.
Before being carried out on the ISS, workflows are also tested on NEEMO (NASA Extreme Environments Mission Operations). NEEMO is a habitat owned by Florida International University that sits on the ocean floor. It’s just 62 feet below the water surface, but it’s still considered an extreme environment because the aquanauts cannot quickly return; they are under full nitrogen saturation and would need to go through decompression to return. Many of the astronauts who visit the ISS (59, in fact) have also spent time in an extreme underwater environment (either NEEMO or the very first SEALAB).
What microorganisms are found on the ISS?
Microbes growing on the ISS are organisms that are typically found in the Earth environment, but when using culture-only identification methods, are also organisms that will grow on the specific media and conditions that are provided for spaceflight culturing. Therefore, it’s a little biased. Using these historical techniques, Staphylococcus and Penicillium are found, along with other organisms associated with human-occupied spaces.
Astronauts have compared what they can identify using the culture method vs. sequencing and analysis. The culture method, as mentioned above, biases towards the organisms that grow on the media they have access to, the temperature and oxygen levels. It plateaus out – they can only identify a certain number of organisms using this methodology. Using the MinION, they see a broader range of organisms. They still identify the microorganisms that grow on the culture media (Staphylococcus, Bacillus, Micrococcus, etc.) in the molecular data, but they also see more of the gram-negative and anaerobic organisms that exist in the human microbiome that they have previously not been able to culture. So, in a nutshell, they are getting a more authentic picture of the human biome in space. For example, in the eating area of the ISS, they find organisms that make up part of the oral microbiome. Air vents collect skin cells, so they find skin-associated microbes there.
Current areas of interest involve developing methods for air, water and fungal analysis using a molecular approach.
What are the unknowns as we venture further?
Artemis is the name of the space program designed to return humans to the Moon. The first launch will be uncrewed and loop around the Moon. A few years later, a much smaller space station, named Gateway, will be put into orbit around the Moon. Astronauts would travel to Gateway in a smaller capsule and then use a rover to visit different Moon locations. This would give more access to the Moon than in the past. The ultimate goal is long-term sustainability on the Moon and in Gateway.
When planning for a trip where samples cannot be returned to Earth for analysis, there are plenty of questions that Dr. Wallace and her team ponder:
1. Power source – can they plug in instruments and have the same type of power they have on the ISS? If refrigeration and freezer capabilities are limited, lyophilized/ stable reagent solutions will be vital.
2. Mass and volume – this will be very constrained. Everything that gets packed on the vehicles will have to have critical value. What materials or aspects of the workflow can be miniaturized?
3. Radiation environment – Radiation levels associated with the ISS are understood; however, Gateway will be in a cis-lunar orbit around the Moon, and there will be a different level of radiation. How will the molecular diagnostic equipment and experiments stand up against this level of radiation?
4. Dust – yes, you read correctly…. dust. NASA learned previously that moon dust is extremely fine. It can cover the astronauts’ extravehicular activity (EVA) suits – what impact will this have on the hardware?
5. Uncrewed spacecraft – unlike the ISS, Gateway will not be continuously occupied. Initially, Gateway will be dormant for potentially 11 months of the year. How will this affect the environment, such as lab hardware or a stagnant water system?
Dr. Wallace tells me that while the “ISS is one of the most amazing engineering marvels humans have accomplished,” it is the unknowns of exploration beyond low-Earth orbit that provide the challenge and excitement to continually drive the planning team to find answers. And, we can’t wait to follow their progress.
NEB will not rent, sell or otherwise transfer your data to a third party for monetary consideration. See our Privacy Policy for details. View our Community Guidelines.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. See www.neb.com/trademarks. The use of these products may require you to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
Don’t miss out on our latest NEBinspired blog releases!
- Sign up to receive our e-newsletter
- Download your favorite feed reader and subscribe to our RSS feed
Be a part of NEBinspired! Submit your idea to have it featured in our blog.


