What is a Disulfide Bond (1 of 4)

Script
Disulfide bonds are formed by the oxidation of sulfhydryl groups between two cysteine side chains, resulting in a covalent bond, greatly increasing the stability of the protein. This process occurs during the folding of a protein.
In this schematic animation, cysteine residues are represented as yellow balls, which are brought together by the folding of a protein, allowing for the formation of a disulfide bond.
If we look at the process in more detail, an electron acceptor is required for the formation of a covalent bond between the two thiol side chains. This process can happen slowly, in the presence of any electron acceptor, such as oxygen, or can be catalyzed by an enzyme that accepts electrons.
Related Videos
-
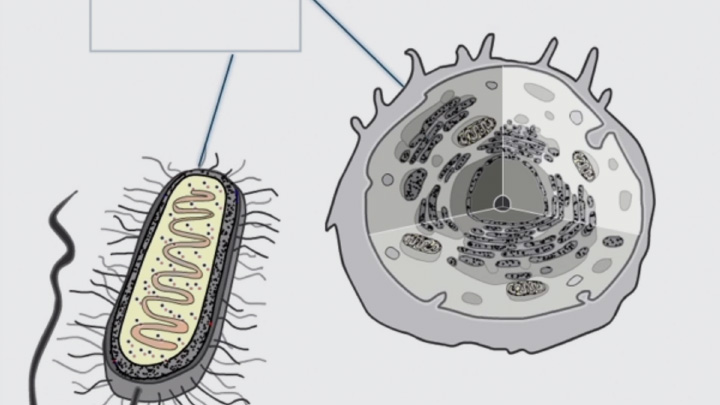
Disulfide bond formation is compartmentalized (2 of 4) -
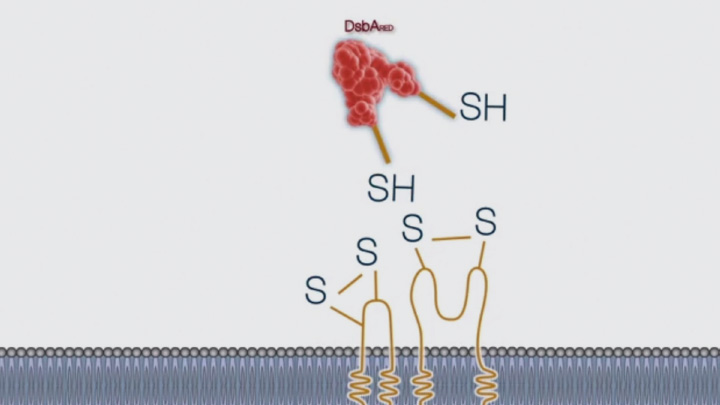
Disulfide bond formation in the periplasm of E. coli (3 of 4) -
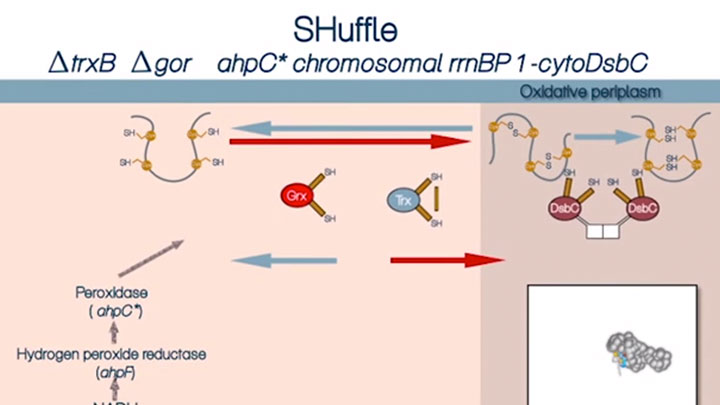
Disulfide bond formation in the cytoplasm of SHUFFLE® cells (4 of 4)

