Combating Neglected Diseases - a genomic approach to identify potential drug targets
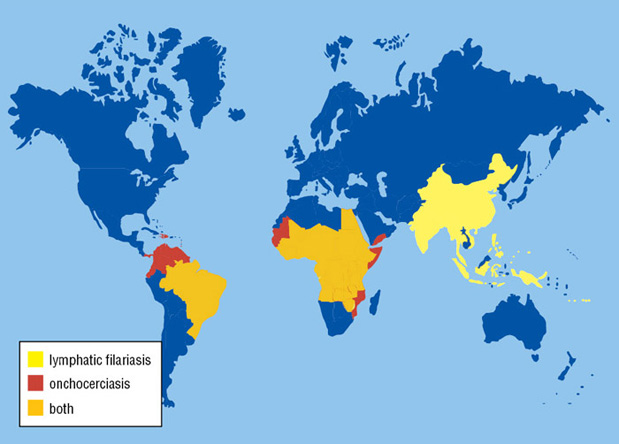
Lymphatic filariasis and onchocerciasis are tropical diseases caused by filarial parasites that are transmitted to humans by insects. Collectively, they afflict around 150 million people in over 80 countries (Figure 1) with more than 1.5 billion at risk of infection (1). In lymphatic filariasis, filarial nematodes such as Wuchereria bancrofti and Brugia malayi (Figure 2), take up residence in the lymphatic system where the thread-like adult worms live and reproduce for almost a decade, spawning millions of immature worms into the blood. The cycle continues when a female anopheline or culicine mosquito ingests the blood containing the worms. Clogged lymphatic ducts lead to severe swelling of limbs and genitalia, as well as damage to kidneys and the lymphatic system itself. In the later stages of infection, the disease is characterized by a disfiguring condition known as elephantiasis resulting in physical disability, severe social stigma and psychological distress (Figure 3). The World Health Organization estimates that lymphatic filariasis ranks third among the infectious diseases in terms of disability, after malaria and tuberculosis. A closely related filarial nematode, Onchocerca volvulus, the causative agent of onchocerciasis or River Blindness, is responsible for the second leading cause (after trachoma) of infectious blindness worldwide. O. volvulus adult worms are also long-lived, and are found in subcutaneous tissues. This species produces immature worms that migrate to the skin and eyes resulting in severe skin pathology and eye lesions. The vectors for O. volvulus are Simulium spp. blackflies that breed in fast flowing rivers.

Despite the severity of filarial disease and its impediment to progress in developing countries, research in this area is neglected and under-funded. There are no vaccines, while drug treatments such as ivermectin, albendazole, and diethylcarbamazine target the immature stages but not the long-lived adult worms. With the real threat of emerging drug resistance resulting from continued reliance on the limited arsenal of drugs that is available, a wider array of choices for drug targets will be invaluable in combating lymphatic filariasis and onchocerciasis.
For more than 20 years, New England Biolabs, Inc. has conducted basic research in molecular parasitology, and collaborated with the World Health Organization and researchers from other institutions and universities. Several laboratories at New England Biolabs are actively engaged in research aimed at identifying essential biological processes and molecules that can be used as targets in high throughput drug discovery pipelines. Much of that effort utilizes genomic sequence data as a core element.
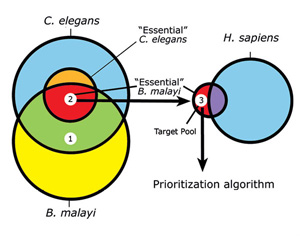
We and others have recently developed an in silico approach for discovering new filarial drug targets in which comparative sequence analysis and functional genomics data from the related model nematode Caenorhabditis elegans are combined into subtractive filters that can be used to identify potentially essential nematode genes and generate a pool of pre-validated candidate targets (2,3). Based on orthology assignments between B. malayi and C. elegans, the essentiality of the B. malayi genes can be inferred from the wealth of data from RNA interference (RNAi) experiments and other functional studies in C. elegans. RNAi, which serves as a technique to examine gene function, has been applied genome-wide in C. elegans, covering approximately 96% of its genes (reviewed in [4]). At present, there are over 60,000 records publicly available from WormBase (www.wormbase.org) reporting RNAi-generated phenotypes in C. elegans. Presumably, orthologs of these genes in B. malayi are also essential, as long as redundant pathways are not present.
Comparative sequence analysis can also be used to eliminate genes with possible mammalian orthologs. Combined with other measures of ‘druggability’, prioritization algorithms can be applied to the remaining set to produce a manageable list of targets for experimental validation (Figure 4).
In the absence of complete genomic sequence, the analysis so far has been limited to a collection of 400,000 nematode ESTs (2,3,5). Our next goal is to apply the same approach to the completed draft genomic sequence of B. malayi, which is expected to be released in the near future (6). A preliminary scan of the pre-release genome sequence data (www.tigr.org) using the method described predicts that a wide range of new potential drug targets will be uncovered. Nonetheless, even utilizing the current EST data set, the applicability of the bioinformatics approach was supported by the identification of known potential nematode drug targets such as chitin synthase (7,8,9), fatty acid desaturase (10) and cofactor independent phosphoglycerate mutase (iPGM) (11,12).
Phosphoglycerate mutase catalyzes the conversion of 2- and 3-phosphoglycerate in the glycolytic and gluconeogenic pathways. Normally, a highly conserved process such as glycolysis would not be considered a fruitful area for identifying selective drug targets. However, PGMs exist in two distinct forms: cofactor-dependent (dPGM) and cofactor-independent (iPGM). The two types possess different molecular weights, share no sequence similarity, have different tertiary structures and even use dissimilar catalytic mechanisms. iPGM serves as a potential filarial drug target because it is widely distributed in nematodes but completely absent from vertebrates; the latter exclusively utilize the dPGM form (12).
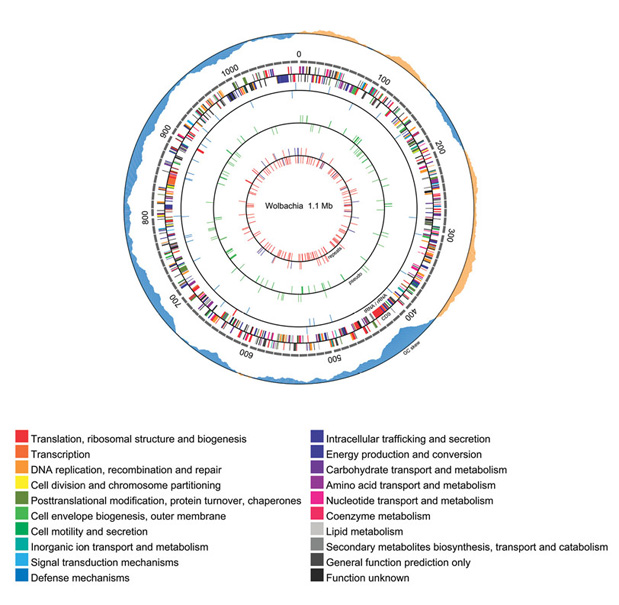
Analysis of potentially essential targets in B. malayi is not confined to the B. malayi genome. In fact B. malayi, W. bancrofti and O. volvulus each contain three genomes: their own chromosomal DNA, a mitochondrial genome and the genome of an intracellular obligate alpha-proteobacterial endosymbiont called Wolbachia (13). In 2005, the 1 Mb genome sequence of Wolbachia, the mutualistic endosymbiont that resides in the cells of B. malayi, was reported by NEB scientists (13), (Figure 5). The attractiveness of Wolbachia as an anti-filarial target is based on the obligate nature of the Brugia-Wolbachia interaction, and the demonstration that the antibiotic doxycycline severely affects development of worms in both laboratory animals and human trials (reviewed in [14]). Recently comparative genomic sequence information has been used to identify its metabolic pathways and predict potential drug targets. Based on the known pathways in B. malayi, the Wolbachia genome analysis suggested that the endosymbiont might supply its host with a number of important nutrients (13).
The genome sequence of nematodes and their endosymbionts represent a valuable resource in combating filarial diseases in the genomic era. The key to unlocking the identity of potential new drug targets is bioinformatic analysis. Validation requires a comprehensive study of the role of the target molecule in the worm and/or endosymbiont. Further development of the most promising targets will hopefully lead to the identification of new lead compounds.
The approach described for identifying new filarial drug targets is applicable to a wide variety of sequenced pathogens, ranging from microbial species to the metazoan parasite analyzed in our study. Given the rapid pace of technological advancements in high throughput genomics, scientists at NEB expect that the methodology will gain widespread applicability.
References
- World Health Organization, “TDR Strategic Direction: Lymphatic Filariasis”, www.who.int/tdr/diseases/lymphfil/direction.htm (2002).
- McCarter, J.P. (2004) Trends Parasitol. 20, 462–468.
- Foster, J.M., et al. (2005) Trends Parasitol. 21, 101–104.
- Mitreva, M., et al. (2005) Trends Genet. 21, 573–81.
- Behm, C.A. et al. (2005) Trends Parasitol. 21, 97–100.
- Ghedin E. et al., Science, in press.
- Harris M.T. et al. (2000) Mol. Biochem. Parasitol. 111, 351.
- Foster, J.M., et al. (2005) Mol. Biochem. Parasitol. 142, 126–132.
- Zhang, Y. et al. (2005) Dev. Biol. 285, 330.
- Watts J.L. and Browse, J. (2002). Proc. Natl. Acad. Sci. USA, 99, 5854–5859.
- Fraser, H.I., Kvaratskhelia, M., and White, M.F. (1999) FEBS Lett. 455, 344–348.
- Zhang, Y. et al. (2004) J. Biol. Chem. 279, 37185.
- Foster J.M. et al. (2005) PloS Biology, 3, 599–614.
- Pfarr, K. and Hoerauf, A. (2006) Mini-reviews in Medicinal Chemistry, 6, 203–210.

