siRNA Transfection Protocol for TransPass R1
Introduction
The following protocol is given with amounts for a 12-well plate format. Use Table 1 to adjust the reagent volumes for other size plates.
Protocol
- Plate cells in complete growth medium containing 10% serum and no antibiotics/antimycotics at an appropriate density, so that they will reach 70-80% cell density (plasmid transfection) or 40-60% (siRNA alone or plasmid and siRNA transfection) at the time of transfection.
- Add 2-6 µl of TransPass R1 siRNA transfection reagent to 100 µl of serum-free medium (e.g., High Glucose DMEM) in a sterile tube and mix by vortexing. Incubate at room temperature for 10-20 minutes.
- Add 0.1–1 µl of siRNA1 to the diluted transfection reagent, mix gently by pipetting and incubate at room temperature for 10–20 minutes to form the transfection complexes.
- Dilute the mixture with 500 µl of complete culture medium (e.g., 10% FBS-containing DMEM).
- Aspirate the culture medium from the cells and immediately replace with the diluted transfection mixture. Evenly disperse the siRNA complexes by gently rocking the plate.
- Return the plate to the incubator and incubate cells overnight.
- Replace the transfection medium with complete medium containing serum and incubate 24-72 hours before assaying target gene inhibition.
For a stock of siRNA at 10 µM, the final concentration of siRNA will be 2.5–25 nM.
Typical cell incubation time points for detecting target gene inhibition are 24–48 hours for mRNA and 48–72 hours for protein.
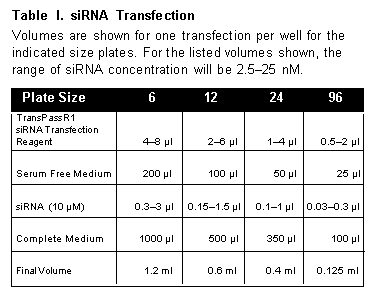
Volumes are shown for one transfection per well for the indicated size plates. For the listed volumes shown, the range of siRNA concentration will be 2.5–25 nM.

