CLIP-Biotin
CLIP-Biotin is suitable for applications such as biotinylation of CLIP-tag™ fusion proteins in living cells for detection with streptavidin fluorophore conjugates or labeling in solution for analysis by SDS-PAGE/Western blot or for capture with streptavidin for binding and interaction studies.
- It is a cell-permeable substrate (BC-Biotin)
- It is based on biotin with an amidocaproyl linker

-
Product Information
This package contains 50 nmol of CLIP-Biotin substrate, sufficient to make 10 ml of a 5 μM solution for the labeling of CLIP-tag fusion proteins in cells.
CLIP-tag is a novel tool for protein research, allowing the specific, covalent attachment of virtually any molecule to a protein of interest. CLIP-tag is a small protein based on mammalian O6-alkylguanine-DNA-alkyltransferase (AGT). CLIP-tag substrates are derivatives of benzylcytosine (BC). In the labeling reaction, the substituted benzyl group of the substrate is covalently attached to the reactive cysteine of CLIP-tag forming a stable thioether link. Although CLIP-tag is based on the same protein as SNAP-tag®, the benzylcytosine substrates form a separate class of substrates, different from the benzylguanine substrates recognized by SNAP-tag. CLIP-tag and SNAP-tag can be used for orthogonal simultaneous labeling.
There are two steps to using this system: subcloning and expression of the protein of interest as a CLIP-tag fusion, and labeling of the fusion with the CLIP-tag substrate of choice. Expression of CLIP-tag fusion proteins is described in the documentation supplied with CLIP-tag plasmids. The labeling of fusion proteins with CLIP-Biotin is described below.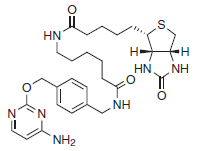
Figure 1: Structure of CLIP-Biotin (MW 569.7 g/mol) - This product is related to the following categories:
- Biotin Labels Products,
- CLIP-tag™ Substrates Products,
- Protein Labeling Products
- This product can be used in the following applications:
- CLIP Cell,
- In vivo Imaging,
- Protein Localization, Protein Labeling Snap Clip
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.




