Furin
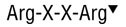
Furin is a recombinant, ubiquitous subtilisin-like proprotein convertase with a minimal cleavage site of Arg-X-X-Arg˅. However, the enzyme prefers the site Arg-X-Lys/Arg-Arg˅.
- It is the major processing enzyme of the secretory pathway and is localized in the trans-golgi network
- Substrates of Furin include blood clotting factors, serum proteins and growth factor receptors such as the insulin-like growth factor receptor

-
Product Information
Furin is a ubiquitous subtilisin-like proprotein convertase. It is the major processing enzyme of the secretory pathway and is localized in the trans-golgi network (1,2). Substrates of Furin include blood clotting factors, serum proteins and growth factor receptors such as the insulin-like growth factor receptor. The minimal cleavage site is Arg-X-X-Arg'. However, the enzyme prefers the site Arg-X-(Lys/Arg)-Arg'. An additional arginine at the P6 position appears to enhance cleavage (3). Furin is inhibited by EGTA, α1- Antitrypsin Portland (4) and polyarginine compounds (5). Product Source
Isolated from Spodoptera frugiperda (Sf9) cells infected with recombinant baculovirus carrying truncated human furin.- This product is related to the following categories:
- Proteases Products
- This product can be used in the following applications:
- Fusion Protein Cleavage,
- Protein Purification,
- Protein Digestion
-
Tools & Resources
-
Citations & Technical Literature
-
Quality, Safety & Legal
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.