O-Glycosidase
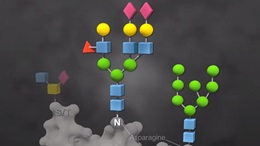
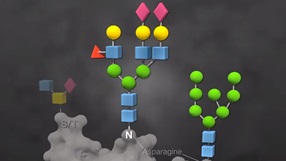
O-Glycosidase, also known as Endo-α-N-Acetylgalactosaminidase, catalyzes the removal of Core 1 and Core 3 O-linked disaccharides from glycoproteins.
- Can be used in conjunction with other exoglycosidases to remove more complex O-glycans
- Recombinant enzyme with no detectable exoglycosidase or other endoglycosidase contaminating activities
- Glycerol -free for optimal performance in HPLC and mass spectrometry analysis
- ≥95% purity, as determined by SDS-PAGE and intact ESI-MS
- Optimal activity and stability for up to 24 months
Featured Video
-
Product Information
O-Glycosidase, also known as Endo-α-N-Acetylgalactosaminidase, catalyzes the removal of Core 1 and Core 3 O-linked disaccharides from glycoproteins.
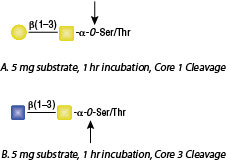
Substrate Specificity:
Product Source
Cloned from Enterococcus faecalis and expressed in E. coli (1).- This product is related to the following categories:
- Endoglycosidases Products,
- Proteome Analysis Products
- This product can be used in the following applications:
- Expression Systems,
- Protein Digestion,
- Glycan Sequencing,
- Recombinant Glycoprotein Expression, Glycoprotein Analysis
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.