NEB® 5-alpha Competent E. coli (Subcloning Efficiency)
Having trouble opening your tubes? NEB Tube Opener can help!
NEB 5-alpha Competent E. coli is a derivative of the popular DH5α. It is T1 phage resistant and endA deficient for high-quality plasmid preparations.
- Ideal for subcloning efficiency transformations, such as plasmid transformation or routine subcloning
- No dry ice surcharge on competent cell shipments
- Free of animal products
- High efficiency chemical and electrocompetent formats are also available
Featured Video
-
Product Information
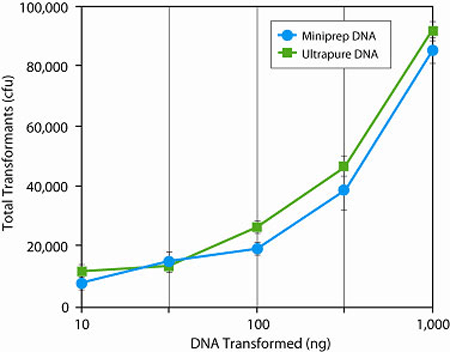
Chemically competent E. coli cells suitable for subcloning efficiency transformations, such as plasmid transformation or routine subcloning (e.g,. inserting a 1 kb fragment into a 2 kb vector). The efficiency of these cells is not sufficiently high for most other cloning applications. For that, we recommend NEB® 5-alpha Competent E. coli (High Efficiency) (NEB #C2987) which has 1,000-fold higher transformation efficiency. Highlights
- Activity of nonspecific endonuclease I (endA1) eliminated for highest quality plasmid preparations
- Efficient transformation of unmethylated DNA derived from PCR, cDNA and many other sources (hsdR)
- Reduced recombination of cloned DNA (recA1)
- Resistance to phage T1 (fhuA2)
- Suitable for blue/white screening by α-complementation of the β-galactosidase gene
- K12 Strain
- DH5α™ derivative
- Free of animal products
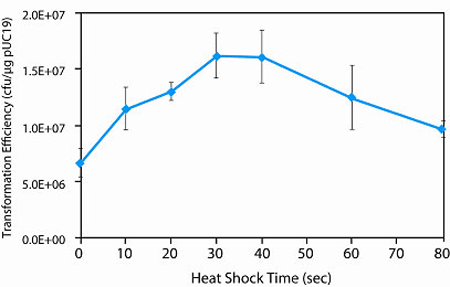
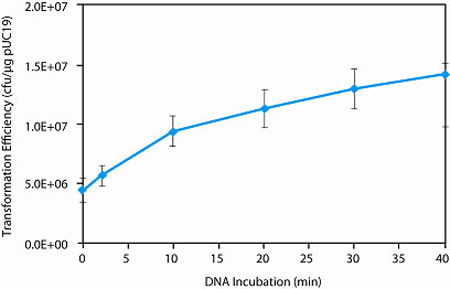
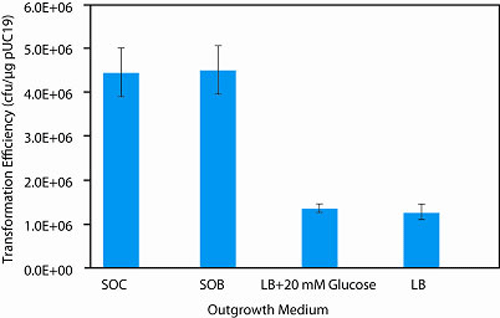
Transformation Efficiency
> 1 x 106 cfu/µg pUC19 DNAGenotype
fhuA2Δ(argF-lacZ)U169 phoA glnV44 Φ80Δ(lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17- This product is related to the following categories:
- Cloning Competent Cell Strains Products
- This product can be used in the following applications:
- Transformation
-
Protocols, Manuals & Usage
-
Tools & Resources
-
FAQs & Troubleshooting
-
Citations & Technical Literature
-
Quality, Safety & Legal
Featured Videos
Other Products You May Be Interested In
Ineligible item added to cart
Based on your Freezer Program type, you are trying to add a product to your cart that is either not allowed or not allowed with the existing contents of your cart. Please review and update your order accordingly If you have any questions, please contact Customer Service at freezers@neb.com or 1-800-632-5227 x 8.