Using aptamers to control enzyme activities: Hot Start Taq and beyond
by Nicole M. Nichols, Ph.D. and Nathan A. Tanner, Ph.D., New England Biolabs, Inc.
As molecular biology and diagnostic applications have become more demanding and sensitive, the ability to control enzymatic activities, and therefore reaction specificity, has naturally followed. Biotechnological innovations have led to several “hot start” approaches to control of DNA polymerases, enabling the spread of PCR from the lab to the clinic with precise enzyme activation. Though powerful, these traditional methods typically require long activation times and very high temperature incubations that are incompatible with essential enzymes like reverse transcriptases. The aptamer-based hot start approach involves the selection of specific, modified aptamers for control of any enzymatic target of interest, even those that can’t tolerate the extreme temperatures of PCR. NEB’s selection of aptamers targeted to RTs and isothermal polymerases has enabled the first “warm start” enzymes that can be used in RT-qPCR and isothermal amplification. Control of enzyme activities remains a critical feature for demanding nucleic acid amplification methods, and the use of aptamer technology brings the benefits of hot start/warm start enzymes to the next generation of rapid, sensitive, and even isothermal molecular tests.
The Polymerase Chain Reaction
The polymerase chain reaction (PCR) is a widely used technique, and the foundation of numerous diagnostic applications that seek to detect minute amounts of DNA via exponential amplification. Successful PCR requires a number of components, including a DNA polymerase capable of tolerating high temperature incubations (94°C or higher) that occur during a typical thermal cycling protocol. Taq DNA Polymerase, originally isolated from Thermus aquaticus, is most commonly used in PCR assays (1). In the early stages of PCR development, it became clear that reaction specificity impacted experimental success (2). Like many non-proofreading Family A DNA polymerases, Taq Polymerase possesses the ability to add bases onto the end of ssDNA in a non-template-dependent manner (i.e., terminal transferase activity). This activity is present, even at room temperature, and can result in the addition of non-specific bases onto the ends of DNA primers in the reaction, enabling them to bind to undesired locations on the DNA and reducing overall reaction specificity. In contrast, at higher temperatures, nonspecific binding is reduced as annealing becomes more stringent. As PCR applications became more complex and demanding, preventing this low temperature activity became critically important to increasing reaction specificity, and numerous techniques and methods have been employed to achieve this protection.
Increasing Reaction Specificity
Early methods focused on the exclusion of key reaction components to mitigate undesired activity at low temperature. As the temperature was increased, any missing components (e.g., polymerase, cofactor) could be spiked into the mixture, triggering the reaction under a more restrictive, high temperature condition – a so-called “hot start” reaction. This method worked, but required the user to open reaction tubes and make very small volume additions, a labor-intensive and contamination-prone process. Subsequent methods aimed to improve upon this approach included sequestering reaction components with wax layers or beads (3). This was also effective, but was not widely adopted by the research community.
The next generation of hot start methods, still in use today, didn’t involve physical removal or exclusion, but instead focused on covalent and non-covalent enzyme interactions or modifications to block activity. The most common of these methods involved the development of an antibody specific to Taq Polymerase, which renders Taq inactive at room temperature, but denatures and dissociates from the enzyme after the initial, high-temperature denaturation step (4,5). Antibody-based Hot Start Taq (Ab-Taq) addressed many of the concerns with prior approaches, and soon became a popular option for anyone looking to increase reaction specificity. However, original Ab-Taq offerings utilized animal-derived antibodies (increasing the possibility of reaction contamination) and were limited in commercial practice to those with a license; it was not surprising that other solutions continued to appear in the marketplace. The most successful of these alternatives was the use of chemicals to reversibly modify amino acid side chains of the polymerase (6). Initial work in this area yielded effective results, but came at a cost: the enzyme could clearly be inactivated but required long activation times at very high temperatures to reactivate, and even then, only a fraction of the initial protein activity could be restored. Advances in this approach, namely changes to the covalent side chain modifications, have resulted in improved versions of chemically-based Hot Start Taq (Chem- Taq), with shorter activation times (4 minutes instead of 10– 15 minutes). The increased potential of inducing DNA damage and the persistent need to add significant amounts of protein may have kept this approach from becoming more widely employed.
Aptamer-Based Enzyme Control Delivers Additional Improvements
A number of years ago, NEB investigated the use of aptamers to impart hot start activation of our enzymes. Generally, aptamers are engineered oligonucleotides that bind to a specific target molecule through non-covalent interactions. SOMAmers, the aptamer-based technology developed by SomaLogic that we have further adapted for use in our products, include specific nucleobase modifications that can improve inhibition profiles and/or reduce unintended side effects (7,8). As with previously-designed Taq inhibitors, the Taq aptamer evolved and engineered by NEB also inhibited polymerase activity at room temperature. This function can be monitored by a variety of assays that include read-outs not just for polymerase activity, but also for reaction specificity. For example, one assay includes an excess of off-target DNA in the reaction and results in the production of multiple amplicons in the presence of non-Hot Start Taq, but only a single, target product in the presence of the aptamer-based Hot Start Taq (NEB-HS Taq). Another assay employed at NEB includes two primers that contain three complementary bases at the 3´ end of each primer. These “poor” primers can serve as a substrate for Taq, and even in the presence of additional input DNA, can generate a clear primer-dimer product in the presence of Taq alone. However, in the presence of NEB-HS Taq, only the desired target-specific product is observed (Figure 1).
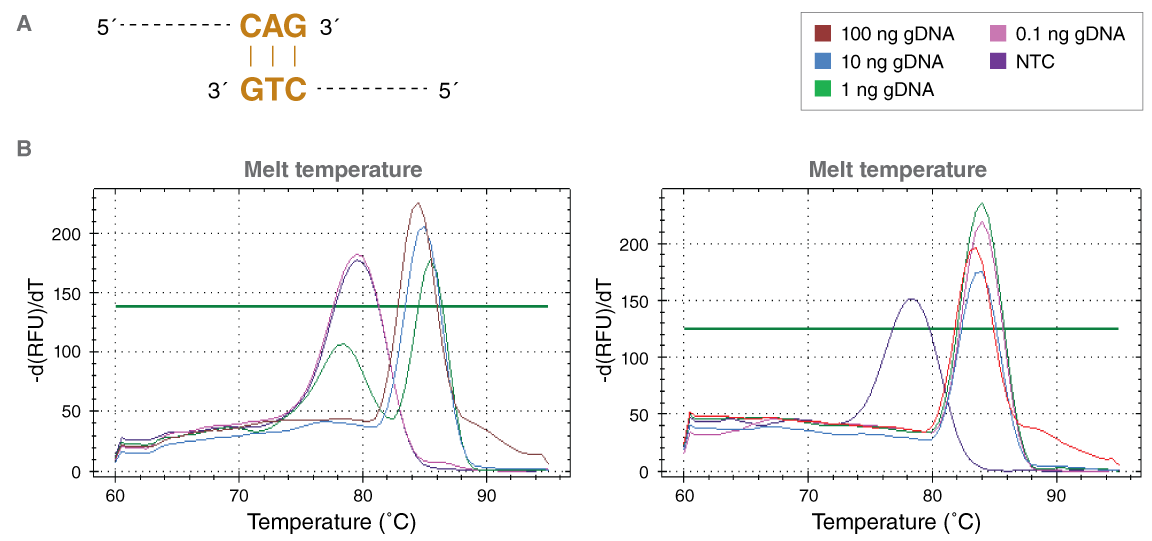
B. Post reaction melt-temp analysis demonstrates the presence of the non specific product only in the absence of template (NTC) for the Hot Start Taq reactions (purple curve, right) but reaction specificity is observed at all other inputs. However, in the absence of a hot start mechanism (left), primerdimer product can be observed with two lower input concentrations as well as in the NTC sample. Reactions (25 μl) contained 0.2 μM primers.
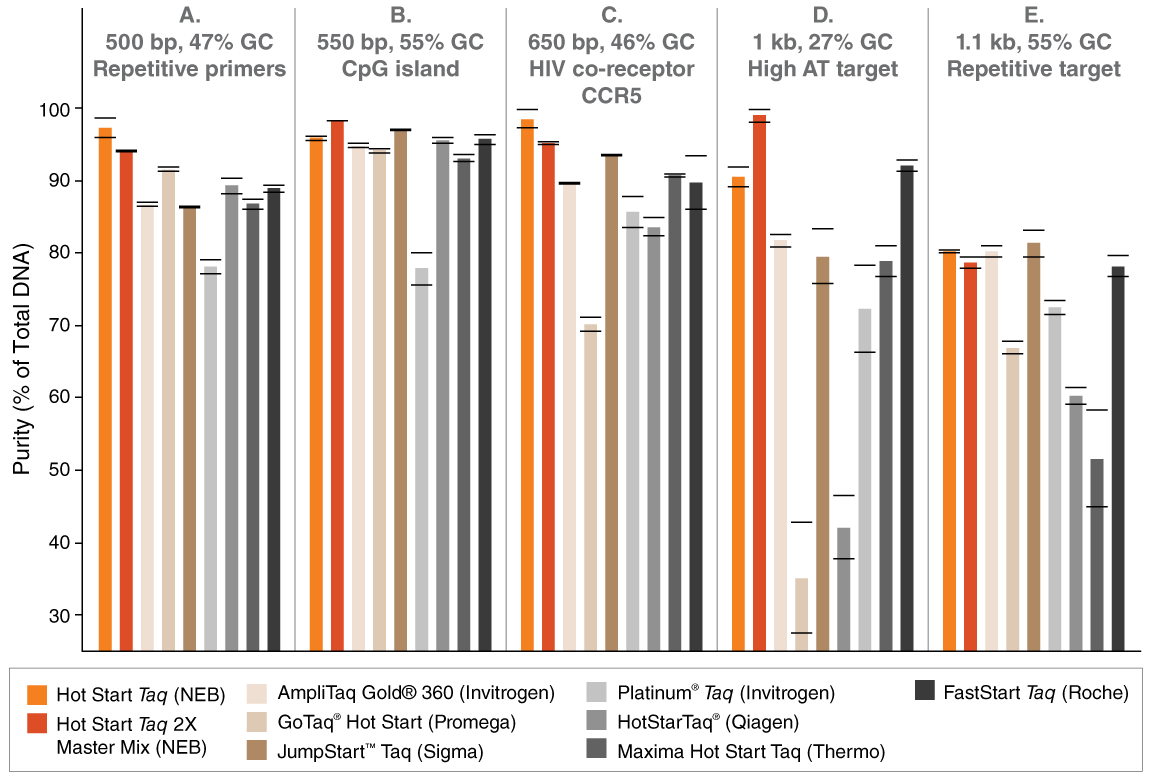
Although the main function of an aptamer-based inhibitor is similar to other hot start mechanisms, there are some key differences between NEB-HS Taq and either Chem- Taq or Ab- Taq. First, unlike other methods, the aptamer-based inhibition/activation process is fully reversible. At the end of thermal cycling, when the temperature of the reaction is decreased, the aptamer rebinds to Taq Polymerase, inhibiting any further activity in the sample. This has proven to be important in workflows where undesired polymerase activity after reaction completion can disturb baseline readings of negative samples, such as in workflows that include a significant delay between interrogation of the first and the last samples of a set (e.g., strip-tubes, 96-well plates, or droplet digital PCR). The second major difference lies in the release of inhibition. Whereas Ab- Taq and Chem- Taq are only activated once the reaction temperature is raised to 94–95°C, the aptamer in NEB-HS Taq dissociates from the polymerase at much lower temperatures (Tm = approximately 45°C), eliminating the need for a specific high temperature activation step, and enabling faster protocols (Table 1). Furthermore, reaction specificity is not impaired (Figure 2). The benefits of an aptamer-based hot start approach can be seen in the numerous NEB products that contain NEB-HS Taq, from the flexible One Taq family of routine PCR products, to the recently-released Luna products that support best-in-class qPCR and RT-qPCR performance.
TABLE 1: Polymerase activation times
| DNA POLYMERASE | HOT START METHOD | ACTIVATION TIME* |
|---|---|---|
| Ampli Taq Gold® 360 (Applied Biosystems) | Chem | 10 min. |
| Platinum® Taq (Invitrogen) | Ab | 30 sec. |
| Hot Start Taq (NEB) | Aptamer | None |
| Go Taq® Hot (Promega) | Ab | 2 min. |
| Hot Star Taq (Qiagen) | Chem | 15 min. |
| FastStart Taq (Roche) | Chem | 4 min. |
| JumpStart™ Taq(Sigma) | Ab | 1 min. |
| Maxima Hot Start Taq (Thermo Fisher Scientific) | Chem | 4 min. |
Extending Aptamer Utility To Isothermal Amplification and Other Enzyme Activities
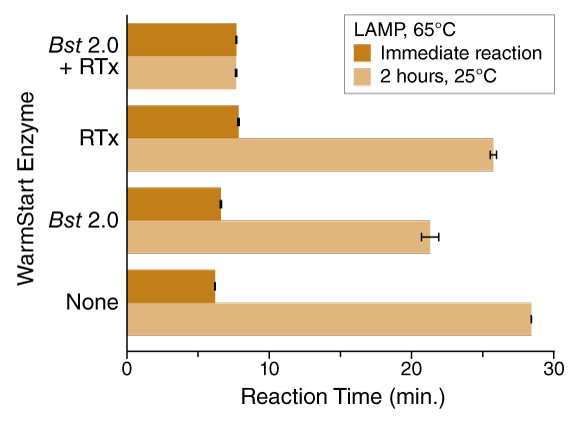
Dissociation at lower temperatures has enabled the use of aptamers for a broader range of polymerases and enzymes, including those that cannot tolerate the high temperatures employed in PCR. For example, aptamers have been particularly useful for isothermal amplification applications, where mesophilic and moderately thermophilic enzymes that catalyze these reactions cannot survive an initial high-temperature denaturation step (and instead use a strong strand displacement activity to separate the DNA duplex). In addition to developing aptamers for an enhanced version of Bst DNA Polymerase (Bst 2.0 WarmStart® DNA Polymerase) to increase specificity in these types of workflows, in 2014 NEB launched the first warm start reverse transcriptase, WarmStart RTx Reverse Transcriptase, specifically for RT-LAMP. Similar to the nonspecific primer extension described above, enzymes utilized in isothermal applications can also give rise to undesired products that affect reaction performance. Reaction conditions, such as very high primer and Mg2+ concentrations, as well as lower optimal temperatures for the enzymes, make isothermal methods especially prone to effects from any off-target activity that occurs during reaction set up. As an example, Figure 3 shows a LAMP reaction where room-temperature incubation results in a significant increase in reaction time (from ~6 minutes to nearly 30 minutes); by utilizing the dual-warm start formulation (WarmStart RTx plus Bst 2.0 WarmStart) the time increase is prevented and consistent reaction performance is maintained. Critically, this protection requires the activities of both enzymes to be controlled.
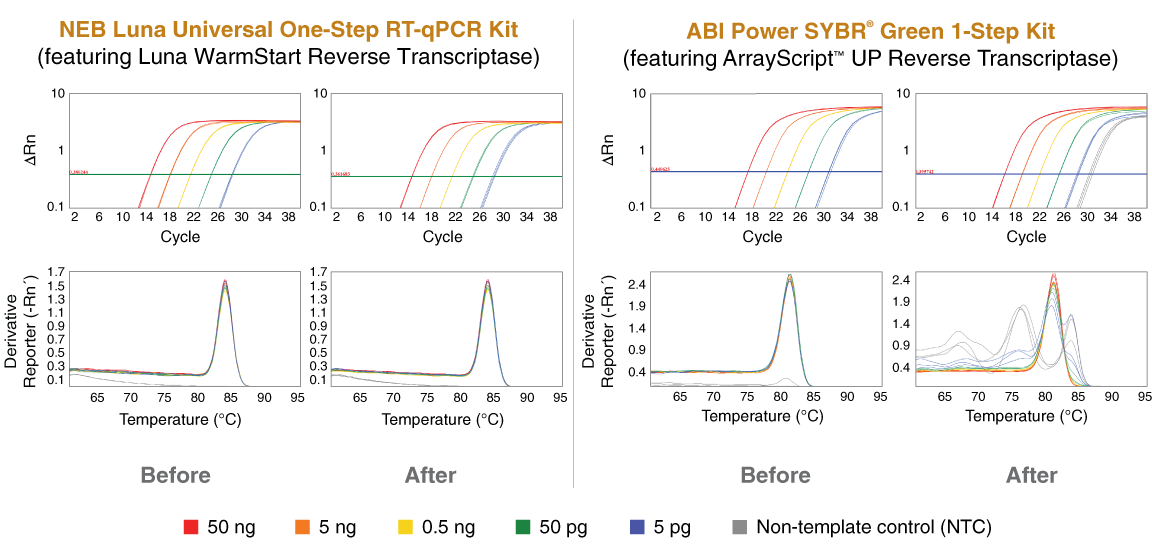
Having observed the utility of a warm start RT in RT-LAMP applications, NEB next focused its efforts on the development and launch of Luna® WarmStart Reverse Transcriptase, to support RT-qPCR applications. As with our previous aptamer development for the DNA-dependent DNA polymerases, these warm start RNA-dependent DNA polymerases (i.e., reverse transcriptases) are inhibited at room temperature, but the aptamer is still released as the temperature is increased, enabling full activity despite the use of moderate reaction temperatures (50–60°C). As with RT-LAMP, it has been through the use of these aptamers that we have been able to identify and prevent RT-mediated non-specific amplification that can occur in certain settings, such as challenging assays or workflows that include a delay between reaction set up and intended initiation. A one-step RT-qPCR example is shown in Figure 4, where a number of reactions were set up simultaneously and half were immediately transferred to a thermocycler and half were left at room temperature for 24 hours. No non-specific amplification can be detected in any of the samples that contained the Luna WarmStart RT. In contrast, clear evidence of non-specific amplification was detected when a more typical RT-qPCR reagent (containing only a Hot Start Taq) was used (Figure 4).
In addition to polymerase activity, aptamers can also be selected to inhibit or moderate other enzymatic activities. For example, the hot start aptamers that were developed for use with Q5® High Fidelity DNA Polymerase were not created to inhibit the polymerase activity, but instead to inhibit its exonuclease activity. Q5 is an engineered polymerase that most closely resembles an archaeal Family B polymerase, and as such it possesses little-to-no detectable 5´→3´ polymerase activity at room temperature. However, Q5 does have a robust 3´→5´ exonuclease (proofreading) activity that enables high-fidelity replication, and this activity remains even at room temperature. This difference in activity temperature profiles compared to Taq (which possesses measureable polymerase activity at room temperature but has no 3´→5´ exonuclease activity at any temperature) can lead to alternate modes of non-specific amplification, thus requiring different solutions (Table 2). Whereas the mechanism of non-specific amplification by Taq is generally via non-templated addition at the 3´ end of the primers, reducing the probability of a Watson-Crick base pair at the desired annealing site, non-specific amplification with a Family B polymerase that possesses proofreading activity appears to occur via exonucleolytic primer degradation at the 3´ end, again reducing specificity at the desired annealing site and increasing the probability of off-target amplification.
TABLE 2: Taq/Q5 polymerase properties
| Taq DNA POLYMERASE | Q5 HIGH-FIDELITY DNA POLYMERASE | |
|---|---|---|
| Polymerase family | A | B |
| 3´→5´ exonuclease* | No | Yes |
| 5´→3´ flap exonuclease** | Yes | No |
| 5´→3´ polymerase (room temperature) | Yes | No |
| Aptamer function | Inhibit polymerase activity at room temperature | Inhibit 3´→5´ exonuclease activity at room temperature |
| Fidelity (relative to Taq)*** | 1 | 280 |
* Also referred to as proofreading activity, this is a key contributor to high fidelity DNA replication in vitro.
** Also referred to as 5´→3´ exonuclease activity, this activity enables the use of hydrolysis (e.g., TaqMan®) probes.
*** Potapov, V. and Ong, J.L. (2017) PLoS ONE. 12(1): e0169774.
Conclusion
NEB’s selection of aptamers enables the ability to inhibit activity at room temperature, and offers unique features that other hot-start approaches cannot. Aptamer-based inhibition is reversible, allowing for an additional level of reaction specificity as the reaction temperature is decreased. Aptamers dissociate rapidly at lower temperatures than traditional hot start methods, eliminating the need for specific activation steps. Additionally, this lower release temperature and the ability to tune release during the aptamer selection process has enabled the creation of “WarmStart” enzymes to bring the benefits of specificity and consistent reaction performance to enzymes outside of typical PCR workflows, such as reverse transcriptases and enzymes used in isothermal amplification methods. Modulating enzyme activity to reduce unwanted side activities remains an important consideration in numerous assays and technologies, and NEB continues to apply its expertise in aptamer chemistry to develop warm and hot start forms of any enzyme where such control is beneficial. But as researchers and developers continue to increase the complexity of these workflows, it is likely that no single hot-start technology will serve all needs. As such, NEB continues to evaluate various methods to ensure that our products enable new and existing applications. With a full understanding of each method’s benefits and limitations, we aim to provide comprehensive hot start reagents for a wide variety of demanding biotechnology applications.
View a PDF of this feature article
References:
- Erlich, H.A., et al. (1988) Nature. 331, 461–462.
- D'Aquila, R.T., et al. (1991) Nucleic Acids Res. 19, 3749.
- Chou, Q., et al. (1992) Nucleic Acids Res. 20, 1717–1723.
- Sharkey, D.J., et al. (1994) Biotechnology. 12, 506–509.
- Kellogg, D.E., et al. (1994) Biotechniques. 16, 1134–1137.
- Birch D.E., et al. Nucleic acid amplification using a reversibly inactivated thermostable enzyme (Patent: US5677152)
- Rohloff, J.C., et al. (2014) Mol Ther Nucleic Acids. 3, e201.
- Gelinas, A.D., et al. (2016) Curr Opin Struct Biol. 36, 122–132.

