COVID-19 Researcher Spotlight: Interview with Greg Patton

Script
Lydia Morrison:
Welcome to the COVID-19 Researcher Spotlight series. Today, we're speaking with Greg Patton, a development scientist here at New England Biolabs. Greg was one of the developers of the SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit, which was just released by NEB. Greg is going to tell us about how the kit works, what type of samples are compatible with it, and he'll explain the kit's intended uses. Hi, Greg. Thanks so much for taking time out of your schedule to join us today.
Greg Patton:
Thanks for having me back on the podcast. Although the circumstances are a little different this time.
Lydia Morrison:
Oh, they certainly are. So thanks for joining me remotely. As one of the developers of any of these new SARS-CoV-2 Rapid Colorimetric LAMP Assay Kit, could you tell our listeners what the kit does?
Greg Patton:
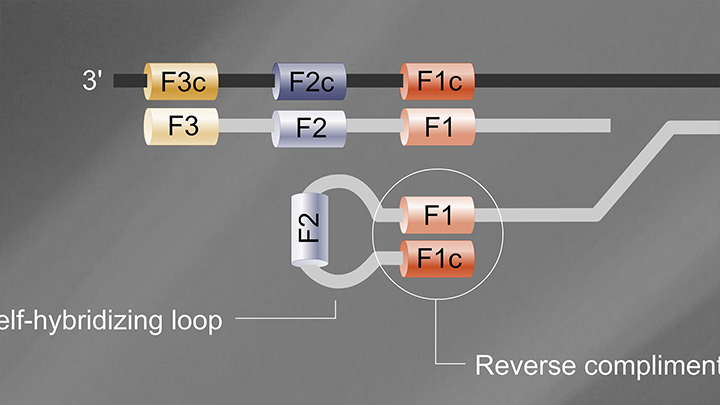
Yeah. So, the kit is a set of reagents designed to determine the presence of SARS-CoV-2 RNA in a sample within 30 minutes, using a simple visual color change. The kit is a research use only kit, and it relies on a loop mediated isothermal amplification or LAMP. LAMP is an isothermal technique that involves four to six primer sets that target a specific nucleic acid and allows amplification of that nucleic acid at one uniform temperature. So, here, the kit uses 65 degrees Celsius.
Greg Patton:
The kit relies on colorimetric detection, and to be able to do that, we take advantage of the chemistry of amplification. So, when a DNA polymerase incorporates a nucleotide, a proton is released in the process. LAMP makes a lot of DNA, which means there are a lot of nucleotides that get incorporated and thus, there are a lot of protons that are released. And so, what we do is we couple the reaction with a pH indicator. So, if there is amplification, you get a generation of a lot of protons. Those protons then basically change the color of the pH indicator. So, if you don't have amplification, the reaction stays pink, but if you have amplification, the reaction changes color to yellow. And so, it allows a very simple way to determine basically the presence of SARS-CoV-2 RNA.
Lydia Morrison:
And how many samples can be tested per kit?
Greg Patton:
Yeah, so the kit is essentially able to test 24 different samples. It's a 96 reaction kit, but we envision four reactions being set up per sample that you want to interrogate. So there's a no template control, a positive control an internal control, and that internal control just makes sure that your sample input is compatible with the assay, and also makes sure that you actually have sample there. And then the final reaction is your SARS-CoV-2 test to determine the presence or absence of SARS-CoV-2 RNA.
Lydia Morrison:
What kind of samples are compatible with the kit?
Greg Patton:
We recommended isolated total nucleic acid going into the kit, but there are a lot of people who are trying to go directly from saliva, for example, and we have tested some of those workflows, and it certainly may work with different sample input types, but there may be some optimization that's required to do some different sample types or go direct.
Lydia Morrison:
Understood. And what does the kit contain?
Greg Patton:
Yeah, so the kit is essentially a six component kit. The first main component is the WarmStart colorimetric LAMP Master Mix with Antarctic Thermolabile UDG and dUTP.
Lydia Morrison:
And what do those kit components do?
Greg Patton:
Yeah, so thermolabile UDG and dUTP enable carryover prevention and carryover prevention is sort of an old technique that prevents previous amplicons from contaminating future reactions, and prevents false positives from occurring. So, basically, amplicons that are made with the carryover prevention mix, so the thermolabile UDG and dUTP end up containing uracil, and then uracil containing amplicons are a substrate for thermolabile UDG. So, any previous amplicons that get carried over into a future reaction will be destroyed by thermolabile UDG during setup. And then thermolabile UDG is quickly inactivated upon basically incubating your reactions at 65 degrees Celsius, which is the isothermal amplification step that drives amplification, so that you can essentially amplify your target nucleic acid without the thermolabile UDG actually destroying what is being amplified.
Lydia Morrison:
Understood. What are the other components of the kit?
Greg Patton:
Yeah, so the second component is a positive control. It is essentially the N gene of a SARS-CoV-2 RNA genome in a plasmid. And it just makes sure that basically you're getting detection of the control as you set up the kit. The next two, basically, reagents are the primers. So there's an internal control primer set that targets the Actin RNA to ensure again that your human sample RNA is present, and make sure that your sample is compatible with the kit.
Greg Patton:
And then the other primer set is the SARS-CoV-2 RNA primer set, which is a mixture of primers that target the N gene and E genes of SARS-CoV-2 RNA.
Lydia Morrison:
And how did we land on these primers?
Greg Patton:
Yeah, so there was a lot of development work that was done on the best primer combo. Really the N and E combined together give us the best sensitivity, and so we settled upon using a dual primer set for the kit. The last two components of the kit are basically water, nuclease-free water to set up the reactions, and then guanidine hydrochloride.
Lydia Morrison:
Why guanidine hydrochloride?
Greg Patton:
We showed in a Biotechniques paper that guanidine hydrochloride actually is an enhancer of LAMP, particularly for our N2 and E1 primer sets that are part of the SARS-CoV-2 primer set, basically 40 to 60 millimolar of guanidine hydrochloride enhances detection. It basically improves sensitivity and gives them more robust yellow color after 30 minutes.
Greg Patton:
And we decided to include that as a separate component, rather than putting it in the master mix itself to give users flexibility. There's a lot of work being done on upfront sample prep workflows. Some of those workflows may include guanidine, and so if a user has a total nucleic acid or RNA direct sample with guanidine present in the solution, they're going to be carrying that into the reaction. And so, we're going to ask users to basically omit the guanidine so that you don't have too much guanidine within the reaction because too much guanidine actually starts to basically cause a loss of performance.
Lydia Morrison:
What makes the kit unique?
Greg Patton:
I think one of the things that makes the kit unique is essentially the minimum amount of equipment you need to basically set up and detect SARS-CoV-2 RNA. Isothermal amplification methods benefit from traditional methods such as RT-qPCR, in that they really just need a heat block. And a set of pipettes to assemble the reactions, and this will really allow this kit to be used in low resource settings.
Greg Patton:
Something like RT-qPCR, you need instrumentation, a real-time instrument to be able to detect the SARS-CoV-2 RNA, and PCR requires a dedicated thermocycler, which draws a lot of power because it has to cycle between temperatures in order to drive amplification, which is in direct contrast to an isothermal method, which you could use a standard heat block or water bath to do the reactions. So, I see the real advantage of the kit being used in low resource settings.
Lydia Morrison:
Understood. So, what are the intended uses of the kit? You mentioned that it's research use only, but obviously there will be interested in using this kit to test patient samples. So, what are the intended use cases for this kit?
Greg Patton:
Yeah, as you mentioned, it's a research use only kit, but certainly someone could take that kit and basically put it in their diagnostic workflow if they were interested in doing so. They would need to collect the relevant clinical data and submit to the FDA in order to get the emergency use authorization on using their workflow containing this kit in diagnosing of patients.
Lydia Morrison:
I see. Were there any particular challenges that arose when you were developing the kit?
Greg Patton:
Yeah. There were a number of challenges during the development of this kit. I think one of them was the rapid timeline in which we needed to develop this product to meet the humanitarian need. I just want to take a moment to thank all of the NEB scientists and our collaborators who have helped get this kit to market. It's really been a collaborative effort over the last few months to make it happen.
Greg Patton:
But I think one of the biggest challenges we faced in the last couple of months was really improving the sensitivity of the kit. So, when we started this project back in March, we had an original primer set, which we called the NA primer set, that we used in a paper that was published in collaboration with the Wuhan Institute of Virology, to basically show proof of principle, that the colorimetric LAMP could be used to use on patient samples and show detection of SARS-CoV-2 RNA.
Greg Patton:
The problem with that original primer set really was it wasn't giving us the best sensitivity. The LOD (limit of detection) of that primer set was probably a couple hundred copies, and that wasn't good enough. The gold standard is RT-qPCR and in those workflows you can generally detect five to 10 copies. So, we spent a lot of time optimizing around the primer sets, and we tested a number of different primer sets. The N2 and E1 primer sets that are now present in the kit individually perform really well. And when we put them together, we really got that synergistic effect, and it allows us to detect down to 50 copies with 95% confidence. So we're claiming the LOD is 50 copies on synthetic RNA.
Lydia Morrison:
That's amazing. So, you mentioned RT-qPCR as being the gold standard of SARS-CoV-2 diagnostics currently. How does this test compare to RT-qPCR in terms of sensitivity?
Greg Patton:
Yeah. So there are limitations of, certainly, colorimetric LAMP. Again, 50 copies is a reasonable LOD. RT-qPCR can go lower, five to 10 copies. But again, you sort of require that dedicated thermocycler to be able to enable amplification and you need some way to determine amplification with RT-qPCR. Usually it's quantitative real-time PCR that's being used, so you need a fluorimeter to monitor the amplification reaction. Here, with colorimetric, the colorimetric LAMP, you essentially get a nice visual readout, no fancy equipment required other than your eyes. And so, it's a quick way to basically get to whether or not SARS-CoV-2 RNA is present.
Lydia Morrison:
Well, I'm really appreciative of all the hard work that you and your team at NEB have put into it, all of the scientists at NEB, as well as the collaborators around the world who have been helping validate the kit and testing the validity of the results. And I think it's really promising. I know a lot of our customers are really excited to see it on the market. So, thank you so much.
Greg Patton:
Yeah. Thank you.
Lydia Morrison:
Thanks joining us for this episode of the COVID-19 Researcher Spotlight Series. If you'd like to learn more about NEB SARS-CoV-2 rapid colorimetric LAMP assay kit, you can visit the product page at neb.com/e2019.
Lydia Morrison:
Hope you can join us for the next COVID-19 Researcher Spotlight, because it's a great one. I'll be interviewing Chris Mason of Weill Cornell Medicine. Chris is a voracious collaborator and has built a consortium of researchers devoting themselves to understanding and detecting SARS-CoV-2. He's also working to bring rapid testing to communities across the United States, as well as to analyze the evolution of SARS-CoV-2 through genome sequencing.
Related Videos
-

COVID-19 Researcher Spotlight: Interview with Nathan Tanner -

NEB® TV Ep. 23 – Colorimetric LAMP in point-of-care diagnostics -
Loop Mediated Isothermal Amplification (LAMP) Tutorial

