Home
Applications
Glycobiology & Proteomics
Recombinant Glycoprotein Expression
O-Glycan Synthesis and Modification

O-Glycan Synthesis and Modification
Return to Recombinant Glycoprotein ExpressionIn mammals, there are a several different types of O-glycosylation. These include mucin and nonmucin O-glycans.
Mucin glycoproteins:
- Tend to be heavily O-glycosylated.
- Are found in mucous secretions and as transmembrane glycoproteins on the cell surface.
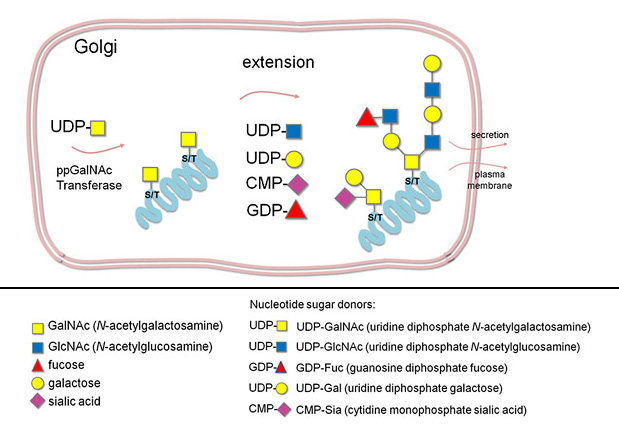
- Addition of O-glycans on the glycoprotein is initiated in the Golgi apparatus.
- Synthesis starts with the addition of an N-acetylgalactosamine (GalNAc) residue to the OH of either a serine or threonine on the glycoprotein.
- There are 8 O-glycan core structures found in mucins (Table 1).
- The growing glycan chain is extended with the addition of other monosaccharides (1, Fig. 1)
| O-glycan | Structure |
|---|---|
| Core 1 or T antigen |  |
| Core 2 |  |
| Core 3 |  |
| Core 4 |  |

Non-mucin glycans are more varied:
- O-fucose and O-glucose residues are:
- transferred to consensus cysteine in certain proteins in the ER
- essential for protein interaction and signal transduction (2)
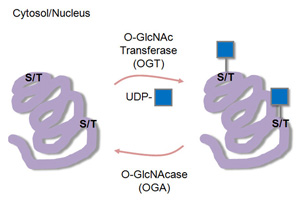
- O-GlcNAc can modify nuclear and cytosolic proteins. This:
- occurs at serine or threonine residues
- is a highly dynamic modification
- plays an important role in cell signaling
- modulates protein function much like phosphorylation (3, Fig. 2)
- can compete directly with phosphate residues for occupancy of serine or threonine residues on the protein
References

