Protocol for use with NEBNext ChIP-seq Library Prep Master Mix Set for Illumina (E6240)
 Colored bullets indicate the cap color of the reagent to be added to a reaction.
Colored bullets indicate the cap color of the reagent to be added to a reaction. Starting Material: 10 ng of chromatin-immunoprecipitated (ChIP) qPCR verified or control DNA, in ≤ 40 μl of water or elution buffer
1.1 End Repair of ChIP DNA
- Mix the following components in a sterile microfuge tube:
ChIP DNA 1–40 μl
 (green) NEBNext End Repair Reaction Buffer (10X) 5 μl
(green) NEBNext End Repair Reaction Buffer (10X) 5 μl
 (green) NEBNext End Repair Enzyme Mix 1 μl
(green) NEBNext End Repair Enzyme Mix 1 μl
Sterile H2O variable
-----------------------------------------------
Total volume 50 μl - Incubate in a thermal cycler for 30 minutes at 20°C.
- Vortex AMPure XP beads to resuspend.
- Add 90 μl (1.8X) of resuspended AMPure XP Beads to the reaction. Mix thoroughly ona vortex mixer or by pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Put the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets.
- Add 200 μl of 80% freshly prepared ethanol to the tube/PCR while in the magnetic stand. Incubate at room temperature for 30 seconds, and then carefully remove and discard the supernatant.
- Repeat Step 5 once.
- Air dry beads for 5 minutes while the tube/PCR plate is on the magnetic stand with the lid open. Caution: Do not overdry the beads. This may result in lower recovery of DNA target.
- Remove the tube/plate from the magnet. Elute DNA target from beads into 50 μl of 0.1X TE.
- Mix well on a vortex mixer or by pipetting up and down and incubate for 2 minutes at room temperature.
- Put the tube/PCR plate in the magnetic stand until the solution is clear. Without disturbing the bead pellet, carefully transfer 44 μl of the supernatant to a fresh, sterile microfuge tube.
- Mix the following components in a sterile tube:
End Repaired, Blunt DNA 44 μl
 (yellow) NEBNext dA-Tailing Reaction Buffer (10X) 5 μl
(yellow) NEBNext dA-Tailing Reaction Buffer (10X) 5 μl
 (yellow) Klenow Fragment (3´→ 5´ exo–) 1 μl
(yellow) Klenow Fragment (3´→ 5´ exo–) 1 μl
-----------------------------------------------
Total volume 50 μl - Incubate in a thermal cycler for 37°C for 30 minutes.
1.4 Clean up Using AMPure XP Beads
- Vortex AMPure XP Beads to resuspend.
- Add 90 μl (1.8X) of resuspended AMPure XP Beads to the reaction. Mix thoroughly on a vortex mixer or by pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Put the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets.
- Add 200 μl of 80% freshly prepared ethanol to the tube/PCR plate while in the magnetic stand. Incubate at room temperature for 30 seconds, and then carefully remove and discard the supernatant.
- Repeat Step 5 once.
- Air dry beads for 5 minutes while the tube/PCR plate is on the magnetic stand with the lid open. Caution: Do not overdry the beads. This may result in lower recovery of DNA target.
- Remove the tube/plate from the magnet. Elute DNA target from beads into 25 μl of 0.1X TE.
- Mix well on a vortex mixer or by pipetting up and down and incubate for 2 minutes at room temperature.
5 Adaptor Ligation of dA-Tailed DNA
Dilute the  (red) NEBNext Adaptor for Illumina* (15 μM) 10-fold in 10 mM Tris-HCI or 10mM Tris-HCI with 10 mM NaCl to a final concentration of 1.5 μM.
(red) NEBNext Adaptor for Illumina* (15 μM) 10-fold in 10 mM Tris-HCI or 10mM Tris-HCI with 10 mM NaCl to a final concentration of 1.5 μM.
- Mix the following components in a sterile microfuge tube.
dA-Tailed DNA 19 μl
 (red) Quick Ligation Reaction Buffer (5X) 6 μl
(red) Quick Ligation Reaction Buffer (5X) 6 μl
Diluted NEBNext Adaptor (1.5 μM) 1 μl
 (red) Quick T4 DNA Ligase 4 μl
(red) Quick T4 DNA Ligase 4 μl
-----------------------------------------------
Total volume 30 μl - Incubate in a thermal cycler for 15 minutes at 20°C.
- Add 3 μl of
 USER enzyme, mix by gently pipetting up and down, and incubate at 37°C for 15 minutes.
USER enzyme, mix by gently pipetting up and down, and incubate at 37°C for 15 minutes.
Note: This step is only required for use with NEBNext Adaptors. USER enxyme can be found in the NEBNext Singleplex (NEB #E7350) or Multiplex (NEB #E7335, #E7500, #E6609 and #E7600) Oligos for Illumina.
![]() A precipitate can form upon thawing of the NEBNext Q5 Hot Start HiFi PCR Master Mix. To ensure optimal performance, place the master mix at room temperature while performing cleanup of adaptor- ligated DNA. Once thawed, gently mix by inverting the tube several times.
A precipitate can form upon thawing of the NEBNext Q5 Hot Start HiFi PCR Master Mix. To ensure optimal performance, place the master mix at room temperature while performing cleanup of adaptor- ligated DNA. Once thawed, gently mix by inverting the tube several times.
1.6 Cleanup of Adaptor Ligated DNA
- Vortex AMPure XP Beads to resuspend
- Add 54 μl of resuspended AMPure XP Beads to the ligation reaction. Mix thoroughly on a vortex mixer or by pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Put the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets.
- Add 200 μl of 80% freshly prepared ethanol to the tube/PCR plate while in the magnetic stand. Incubate at room temperature for 30 seconds, and then carefully remove and discard the supernatant.
- Repeat Step 5 once.
- Air dry beads for 5 minutes while the tube/PCR plate is on the magnetic stand with the lid open. Caution: Do not overdry the beads. This may result in lower recovery of DNA target.
- Remove the tube/plate from the magnet. Elute DNA target from beads into 105 μl of 10 mM Tris-HCI or 0.1 X TE to the beads for bead-based size selection. Note: For size selection using E-Gel size select gels or standard 2% agarose gels, elute the DNA target at desired volume.
- Mix well on a vortex mixer or by pipetting up and down several times, and incubate for 2 minutes at room temperature.
- Put the tube/PCR plate in the magnetic stand until the solution is clear. Transfer 100 μl of supernatant (or desired volume) to a new tube/well, and proceed to bead based size selection.
1.7 Size Selection of Adaptor Ligated DNA Using Agencourt AMPure XP Beads
| Insert Size | 150 bp | 200 bp | 250 bp | 300 bp | 400 bp | 500 bp | 700 bp |
| Total library size (insert + adaptor) |
270 | 320 | 370 | 420 | 530 | 660 | 820 |
| Bead: DNA ratio* 1st bead selection |
0.9X | 0.8X | 0.7X | 0.6X | 0.55X | 0.5X | 0.45X |
| Bead: DNA ratio* 2nd bead selection |
0.2X | 0.2X | 0.2X | 0.2X | 0.15X | 0.15X | 0.15X |
Note: (X) refers to the original sample volume of 100 μl.
- Add 90 μl (0.9X) resuspended AMPure XP Beads to 100 μl DNA solution. Mix well on a vortex mixer or by gently pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Place the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully transfer the supernatant to a new tube/well (Caution: do not discard the supernatant). Discard beads that contain the large fragments.
- Add 20 μl (0.2X) resuspended AMPure XP Beads to the supernatant, mix well and incubate for 5 minutes at room temperature.
- Put the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully remove and discard the supernatant. Be careful not to disturb the beads that contain DNA targets (Caution: do not discard beads).
- Add 200 μl of 80% freshly prepared ethanol to the sample while in the magnetic stand. Incubate at room temperature for 30 seconds, and then carefully remove and discard the supernatant.
- Repeat Step 6 once.
- Air dry beads for 5 minutes while the tube/PCR plate is on the magnetic stand with lid open. Caution: Do not overdry the beads. This may result in lower recovery of DNA target.
- Remove the tube/plate from the magnet. Elute DNA target from the beads by adding 22 μl of 10 mM Tris-HCI or 0.1X TE.
- Mix well on a vortex mixer or by gently pipetting up and down and incubate for 2 minutes at room temperature.
- Put the tube/PCR plate in the magnetic stand until the solution is clear. Without disturbing the bead pellet, carefully transfer 20 μl of the supernatant to a clean PCR tube and proceed to enrichment.
Follow Section 1.8A if you are using the following oligos (10 μM primer):
NEBNext Singleplex Oligos for Illumina (NEB #E7350) lot 0071412
NEBNext Multiplex Oligos for Illumina (Set 1, NEB #E7335) lot 0091412
NEBNext Multiplex Oligos for Illumina (Set 2, NEB #E7500) lot 0071412
NEBNext Multiplex Oligos for Illumina (Dual Index Primers, NEB #E7600) all lots
Follow Section 1.8B if you are using NEBNext Multiplex Oligos for Illumina (96 Index Primers, NEB #E6609).
Follow Section 1.8C if you are using the following oligos (25 μM primer):
NEBNext Singleplex Oligos for Illumina (NEB #E7350 ) lots 0051402 or 0061410
NEBNext Multiplex Oligos for Illumina (Set 1, NEB #E7335) lots 0071402 or 0081407
NEBNext Multiplex Oligos for Illumina (Set 2, NEB #E7500) lots 0051402 or 0061407
1.8A PCR Enrichment of Adaptor Ligated DNA
- Mix the following components in sterile strip tubes: Adaptor Ligated DNA Fragments 20 μl
- PCR cycling conditions:
CYCLE STEP TEMP TIME CYCLES Initial Denaturation 98°C 30 seconds 1 Denaturation
Annealing/ Extension98°C
65°C10 seconds
75 seconds15 Final Extension 65°C 5 minutes 1 Hold 4°C ∞
- Proceed to Cleanup Ampure XP Beads in Section 1.9.
 (blue) Index Primer/ i7 Primer*,** 2.5 μl
(blue) Index Primer/ i7 Primer*,** 2.5 μl (blue) Universal PCR Primer/ i5 Primer*,*** 2.5 μl
(blue) Universal PCR Primer/ i5 Primer*,*** 2.5 μl (blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl
(blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl--------------------------------------------------------------------------------
Total volume 50 μl
* The primers are provided in NEBNext Singleplex (NEB #E7350) or Multiplex (NEB #E7335, #E7500 or #E7600) Oligos for Illumina. For use with Dual Index Primers (NEB #E7600), look at the NEB #E7600 manual for valid barcode combinations and tips for setting up PCR reactions.
** For use with NEBNext Multiplex Oligos (#E7335 or #E7500) use only one Index Primer per PCR reaction. For use with Dual Index Primers (NEB #E7600) use only one i7 Primer per reaction.
*** For use with Dual Index Primers (NEB #E7600) use only one i5 Primer per reaction.
- Mix the following components in sterile strip tubes: Adaptor Ligated DNA Fragments 15 μl
- PCR cycling conditions:
- Proceed to Cleanup Using Ampure XP Beads in Section 1.9.
 (blue) Index/ Universal Primer Mix* 10 μl
(blue) Index/ Universal Primer Mix* 10 μl (blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl
(blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl--------------------------------------------------------------------------------
Total volume 50 μl
* The primers are provided in NEBNext Multiplex Oligos for Illumina, NEB #E6609. Please refer to the NEB #E6609 manual for valid barcode combinations and tips for setting up PCR reactions.
| CYCLE STEP | TEMP | TIME | CYCLES |
| Initial Denaturation | 98°C | 30 seconds | 1 |
| Denaturation Annealing/ Extension |
98°C 65°C |
10 seconds 75 seconds |
2-4* |
| Final Extension | 65°C | 5 minutes | 1 |
| Hold | 4°C | ∞ |
- Mix the following components in sterile strip tubes:
 (blue) Index Primer*, ** 1 μl
(blue) Index Primer*, ** 1 μl (blue) Universal PCR Primerr* 1 μl
(blue) Universal PCR Primerr* 1 μl (blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl
(blue) NEBNext Q5 Hot Start HiFi PCR Master Mix 25 μl--------------------------------------------------------------------------------------
Total volume 50 μl
-
* The primers are provided in NEBNext Singleplex (NEB #E7350) or Multiplex (NEB #E7335 or #E7500) Oligos for Illumina.
- PCR cycling conditions:
- Proceed to Cleanup Using Ampure XP Beads in Section 1.9.
** For use with NEBNext Multiplex Oligos (NEB #E7335 or #E7500) use only one Index Primer per PCR reaction.
| CYCLE STEP | TEMP | TIME | CYCLES |
| Initial Denaturation | 98°C | 30 seconds | 1 |
| Denaturation Annealing/ Extension |
98°C 65°C |
10 seconds 75 seconds |
15 |
| Final Extension | 65°C | 5 minutes | 1 |
| Hold | 4°C | ∞ |
- Vortex AMPure XP Beads to resuspend.
- Add 45 μl (0.9X) of resuspended AMPure XP Beads to the PCR reactions (~50 μl). Mix well on a vortex mixer or by pipetting up and down at least 10 times.
- Incubate for 5 minutes at room temperature.
- Put the tube/PCR plate on an appropriate magnetic stand to separate beads from supernatant. After the solution is clear (about 5 minutes), carefully remove and discard the supernatant. Be careful not to disturb the beads that contain the DNA targets.
- Add 200 μl of freshly prepared 80% ethanol to the tube/PCR plate while in the magnetic stand. Incubate at room temperature for 30 seconds, and then carefully remove and discard the supernatant.
- Repeat Step 5 once.
- Air dry the beads for 5 minutes while the tube/PCR plate is on the magnetic stand with the lid open. Caution: Do not overdry the beads. This may result in lower recovery of DNA target.
- Remove the tube/plate from the magnet. Elute the DNA target from the beads by adding 30 μl of 0.1X TE.
- Mix well on a vortex mixer or by pipetting up an down and incubate for 2 minutes at room temperature.
- Put the tube/PCR plate in the magnetic stand until the solution is clear. Without disturbing the bead pellet, carefully transfer 25 μl of the supernatant to a clean LoBind®(Eppendorf AG) tube. Libraries can be stored at -20°C.
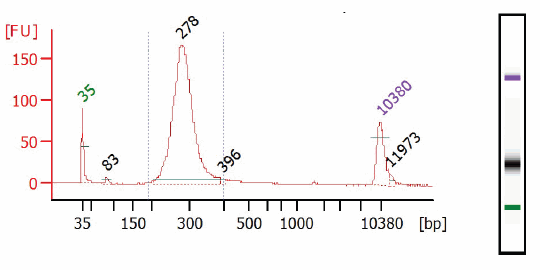
- Dilute 2-3 μl of the library 20 fold with 10 mM Tris-HCI or 0.1X TE and assess the library quality on a Bioanalyzer®(Agilent Technologies, Inc.) high sensitivity chip. Check that the electropherogram shows a narrow distribution with a peak size approximately 275 bp.
Figure 1.1: Bioanalyzer traces of final library.