TransPass R1: Plasmid DNA and siRNA Transfection Protocol
Protocol
- Plate cells in complete growth medium containing 10% serum and no antibiotics/antimycotics at an appropriate density, so that they will reach 70-80% cell density (plasmid transfection) or 40-60% (siRNA alone or plasmid and siRNA transfection) at the time of transfection.
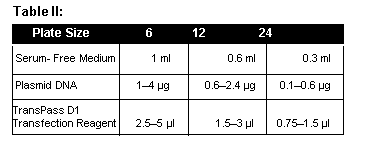
- Mix an appropriate amount of serum-free medium (Table II) with plasmid DNA in a sterile tube and mix by vortexing. Add an appropriate amount of TransPass D1 DNA Transfection Reagent for the size of plate you are using (Table II). Incubate at room temperature for 20 minutes.
- Aspirate culture medium from cells. Wash cells once with serum-free medium. Aspirate the culture medium from the cells and immediately replace with the transfection complex mixture.
- Incubate cells for 3 hours.
- Replace medium with fresh medium containing serum. Incubate cells 2-3 hours.
- During this time prepare the siRNA transfection complex according to protocol 1.
- Aspirate the culture medium from the cells and immediately replace with the diluted siRNA transfection complex mixture (step 4, protocol 1).
- Incubate cells 24–48 hours before analysis.
* Successful DNA transfection has been performed in the following cell lines: COS-7, HeLa, NIH3T3, U2OS, HCT116 and HEK293.

